The digestive system, composed of the oral cavity, esophagus, stomach, duodenum, jejunum, ileum, colon, rectum, anus, liver, gallbladder, bile ducts, and pancreas, contains the largest number of organs in the human body. Diseases affecting these organs are common and often interconnected, with some clinical manifestations overlapping and complex. During the diagnostic and treatment process, characterized by moving from surface to depth, sorting relevant from irrelevant, and discerning truth from misrepresentation, it is essential for medical professionals to possess a solid, continually updated understanding of digestive physiology, biochemistry, pathophysiology, pharmacology, endoscopy, and vascular interventional techniques. Rigorous logical reasoning, extensive knowledge of social and humanities contexts, and skills in patient care are also required. Digestive system diseases often involve critical or life-threatening conditions. In such urgent situations, a doctor's sense of responsibility, physical fitness, sound psychological resilience, and skilled medical expertise are all crucial.
Physiological Antireflux Mechanisms of the Esophagus
During normal swallowing, the lower esophageal sphincter (LES) relaxes to allow food to enter the stomach. Transient LES relaxation can also occur outside of swallowing, causing small and short episodes of gastroesophageal reflux. However, the presence of the following antireflux mechanisms prevents the occurrence of pathological gastroesophageal reflux.
Esophageal-Gastric Antireflux Barrier
This barrier consists of the anatomical structures at the esophagogastric junction, including the LES, the diaphragmatic crura, the phrenoesophageal ligament, and the acute angle between the esophagus and the gastric fundus. The LES, a ring of circular muscle measuring about 3–4 cm in length at the distal end of the esophagus, generates a high-pressure zone at the esophagogastric junction to prevent gastric contents from re-entering the esophagus.
Esophageal Clearance Mechanism
Under normal conditions, when gastroesophageal reflux occurs, most of the refluxate is returned to the stomach through one or two spontaneous or secondary esophageal peristaltic contractions, a process known as esophageal clearance. Remaining refluxate is further neutralized and flushed away by saliva.
Esophageal Mucosal Barrier
After refluxate enters the esophagus, the esophageal mucosa provides defense through its barrier mechanisms, including salivary secretions, stratified squamous epithelium, and submucosal blood supply, which protect the mucosa from damage caused by refluxate.
Gastric Mucosal Barrier
The gastric epithelium forms invaginations, producing gastric glands. Pyloric glands, located in the gastric antrum and pylorus, are highly branched, coiled tubular mucous glands containing numerous endocrine cells. These glands are the main source of mucus and gastrin (also known as gastric secretagogue). Oxyntic glands, located in the gastric fundus and body, have fewer branches and are composed of chief cells, parietal cells, mucous neck cells, and endocrine cells. These glands are the primary producers of gastric acid, pepsinogen, and intrinsic factor; they are also referred to as acid-secreting glands. Cardiac glands, located near the gastric cardia, are single-tubed glands that primarily secrete mucus.
Gastric juice has a pH ranging from approximately 0.9 to 1.5, with normal daily secretion amounts of about 1.5 to 2.5 liters. The acidic environment activates pepsinogen to pepsin. Despite repeated exposure to pathogenic microorganisms, irritating agents, and potentially damaging substances, the gastric mucosa remains intact and functions effectively, maintaining a thousand-fold pH gradient between the gastric lumen and the mucosa. This integrity is supported by three tiers of the gastric mucosal barrier:
Pre-Epithelial Layer
This layer consists of a 0.5-mm-thick gel layer of mucus and bicarbonate covering the surface of the gastric epithelial cells. It prevents damage to the epithelial cells from concentrated hydrochloric acid, pepsin, pathogenic microorganisms, and other irritating or damaging substances in the stomach. It also preserves the high pH gradient between the acidic gastric juice and the neutral mucosa.
Epithelial Cells
The apical membranes and tight junctions of epithelial cells function as barriers to acid back-diffusion and harmful factors in the gastric lumen. The epithelial cells regenerate rapidly, with a turnover time of approximately 2–3 days, facilitating rapid repair following damage. Epithelial cells also produce inflammatory mediators and contain intraepithelial lymphocytes that are critical components of mucosal immunity.
Subepithelial Layer
Gastric mucosal cells have low glycogen reserves and limited energy production under hypoxic conditions. To maintain the integrity of the gastric mucosa, adequate oxygen and nutrients are essential. The extensive capillary network in the gastric mucosa supports the vigorous secretory activity and continuous renewal of epithelial cells, removes locally produced metabolic wastes, and transports back-diffused hydrochloric acid away from the tissue. Healthy gastric mucosal blood circulation plays a vital role in maintaining mucosal integrity. Prostaglandin E demonstrates protective effects on gastric mucosal cells by enhancing mucosal blood flow and promoting mucus and bicarbonate secretion. It is one of the most well-recognized protective molecules for the mucosa.
Secretion and Regulation of Gastric Acid
Food stimulation in the gastric antrum promotes the secretion of gastrin by the G cells in pyloric glands. Gastrin acts on enterochromaffin-like cells (ECL cells) in the gastric body through endocrine and paracrine pathways, stimulating the release of histamine. Histamine and gastrin, by binding to histamine H2 receptors and gastrin receptors on parietal cells, work together to promote the synthesis and secretion of hydrochloric acid by parietal cells. Somatostatin, secreted by D cells in the antrum, exerts a negative regulatory effect on all three cells involved in this process.
The secretion of hydrochloric acid by parietal cells can generally be divided into three main steps:
- Stimulation of specific receptors on parietal cells by histamine, acetylcholine, and gastrin.
- Intracellular production of hydrogen ions in parietal cells mediated by cyclic AMP (cAMP) or calcium ions.
- The action of H+-K+-ATPase, also known as the proton pump, located in the secretory tubules and vesicles of parietal cells, actively transporting hydrogen ions (H+) against the concentration gradient into the gastric lumen.
Additionally, acetylcholine from the enteric nervous system, through neuroendocrine signaling, influences the functional states of parietal cells, G cells, and D cells. Its role in the overall regulation of gastric acid secretion exhibits significant variability.
Intestinal Mucosal Barrier
As the intestines manage an extensive exposure to food and a symbiotic relationship with microorganisms within the intestinal lumen, the barrier defense system functions as a critical mechanism. This system effectively prevents the translocation of commensal bacteria and their metabolic products beyond the intestinal lumen into other tissues and organs, thereby protecting the body from endogenous microorganisms and their toxins. The intestinal mucosal barrier is a structural and functional entity that separates substances within the intestinal lumen from the internal environment of the body, ensuring the stability of the internal environment. It is composed of the mechanical barrier, chemical barrier, immune barrier, biological barrier, and intestinal motility.
Mechanical Barrier
The mechanical barrier is a critical component constituted by the intestinal epithelial cells, the tight junctions between cells, and the bacterial biofilm. It plays the most important role in the intestinal barrier's defensive function.
Chemical Barrier
The chemical barrier includes gastric acid and bile salts, which can deactivate a large number of bacteria entering the intestines via the oral route. It also consists of mucus and digestive fluids secreted by intestinal epithelial cells, as well as antimicrobial substances produced by the normal commensal bacteria in the intestinal lumen.
Immune Barrier
The intestine serves as a key peripheral immune organ in the human body. The immune barrier is composed of gut-associated lymphoid tissue (including intraepithelial lymphocytes, lamina propria lymphocytes, and Peyer’s patches), mesenteric lymph nodes, Kupffer cells in the liver, and secretory antibodies (sIgA) and defensins produced by immune cells. It has important roles in both innate and adaptive immunity.
The innate immunity of the intestinal mucosa is a naturally endowed mechanism of the body. It acts rapidly and employs various defense strategies, though it lacks immunological memory, reacting similarly to repeated stimulations by the same pathogen. Adaptive immunity in the intestine is initiated when specific lymphocytes recognize exogenous antigens, followed by the proliferation and differentiation of lymphocytes into effector cells. Although slower to activate, adaptive immunity is characterized by immunological memory and specificity, enabling it to augment and enhance the functional effects of innate immunity.
Biological Barrier
The detail can be seen in the section of "Intestinal Microecology."
Intestinal Motility
Intestinal motility functions like a scavenger for the gut. In conditions such as intestinal obstruction or paralysis, it is often accompanied by bacterial overgrowth in the small intestine.
Intestinal Microecology
The gut microbiota ecosystem consists of bacteria, fungi, viruses, and their metabolites, collectively referred to as the “second genome of the human body.” The bacteria in the human gut alone exceed 1,000 species and number between 1012 and 1014 organisms, which is ten times the number of human cells. The genes encoded by these gut bacteria amount to approximately 3.3 million, 150 times more than the number of human genes. The ratio of anaerobic bacteria to aerobic bacteria in the intestinal lumen is approximately (100–1,000):1. Anaerobic bacteria rarely translocate and inhibit the growth of potential pathogens, while aerobic bacteria are more likely to translocate and serve as a primary source of endotoxin in circulation.
The gut microbiota can be broadly categorized into the following groups:
- Probiotics: These primarily include anaerobic bacteria such as various species of Bifidobacterium and Lactobacillus. Probiotics are often closely associated with the mucus layer and are essential for human health. They synthesize various vitamins, participate in food digestion, promote intestinal motility, prevent pathogenic bacteria from contacting intestinal epithelial cells, and degrade harmful and toxic substances.
- Opportunistic Pathogens: This category includes bacteria like Escherichia coli and Enterococcus. These bacteria have dual roles; under normal circumstances, they are beneficial to health. However, when their proliferation becomes uncontrolled or when they translocate from the gut to other parts of the body, they can cause diseases.
- Harmful Bacteria: Examples include Shigella and Salmonella. When these bacteria grow excessively, they can cause various diseases or impair the functioning of the immune system.
Factors such as age, sex, genetic heritage, geography and diet, physical activity, and medications influence the gut microbiota ecosystem. Microorganisms and humans have co-evolved, forming a mutually dependent and interdependent symbiotic relationship. Interactions between microbes, as well as between microbes and their host, influence host physiological functions. The intestinal mucosal barrier and gut microbiota have a bidirectional regulatory relationship, mutually influencing each other. Gut microbiota impacts host nutrition, metabolism, immunity, development, and aging. It is notable that the influence of gut microorganisms extends far beyond the gastrointestinal tract, including effects on the gut-liver axis and the brain-gut-microbiota axis.
The functions of gut microbiota include the following:
- Metabolic Function: Gut microbiota secrete complex proteases, possess redox capabilities, and facilitate the breakdown of dietary components. They also metabolize or transform endogenous and exogenous substances.
- Nutritional Function: They synthesize various vitamins, amino acids, peptides, and short-chain fatty acids. Their metabolic products promote the absorption of minerals and nutrients, thereby influencing host nutritional metabolism.
- Host Immune Function: They regulate the development and maturation of the host’s immune organs and act as broad-spectrum antigens to stimulate host immune responses, including both humoral and cellular immunity.
- Intestinal Defense Function: As a crucial component of the intestinal mucosal barrier, they prevent the invasion or colonization of potential pathogens and help maintain the structural and functional integrity of the intestinal mucosal barrier. Specific components of the gut microbiota can modify the expression of tight junction proteins in epithelial cells, and microbial metabolites like butyrate play essential roles in maintaining the epithelial barrier. Microbial degradation and metabolism of bile acids can alter the bile acid pool, such as chenodeoxycholic acid and deoxycholic acid, which act as secretagogues in the colon.
The gut microbiota ecosystem is not only associated with digestive system diseases such as inflammatory bowel disease (IBD), functional gastrointestinal disorders, celiac disease, colorectal cancer, and nonalcoholic fatty liver disease (NAFLD) but also affects numerous chronic diseases, including metabolic disorders like obesity and type 2 diabetes, cardiovascular diseases, neuropsychiatric disorders, immune-related diseases, and malignancies of multiple organs. It even plays a role in modulating the efficacy of certain drug therapies.
Gastrointestinal Peptides
The endocrine cells scattered throughout the gastrointestinal tract produce over 50 types of gastrointestinal peptides, such as cholecystokinin, somatostatin, vasoactive intestinal peptide, and substance P. The digestive tract is recognized as the largest endocrine organ in the body. These gastrointestinal peptides play significant and complex regulatory roles in secretion, motility, substance transport, immunity, and the repair of intestinal epithelial cells within the gastrointestinal system. Additionally, they influence the functions of other organs in the body.
Digestion, Absorption, and Hepatic Metabolism of Major Nutrients
Carbohydrates
Starch from food is hydrolyzed by pancreatic amylase into disaccharides, which are subsequently digested into monosaccharides by disaccharidases located on the brush border of small intestinal epithelial cells. These monosaccharides are then absorbed into the bloodstream from the small intestine. Some of these carbohydrates provide energy for the body, while others are stored as glycogen in the muscle and liver. Muscle glycogen is primarily used to meet urgent energy needs during muscle contraction, whereas liver glycogen plays a crucial role in stabilizing blood glucose levels, which is particularly vital for the brain and red blood cells. When blood glucose levels drop, liver glycogen is broken down into glucose and released into the bloodstream to replenish blood glucose. After fasting for more than 10 hours, most of the stored liver glycogen is depleted, at which point the liver converts some of the body's proteins and fats into glucose and glycogen through gluconeogenesis. Impaired absorption of nutrients in the small intestine can lead to malnutrition, while excessive carbohydrate absorption may contribute to obesity. When the liver is damaged, the synthesis and breakdown of glycogen, as well as gluconeogenesis, become impaired, making it difficult to maintain normal blood glucose levels. Chronic liver disease, as a result, is often accompanied by diabetes.
Lipids
After emulsification by bile salts in the small intestine, lipids are digested by pancreatic lipase into monoglycerides, fatty acids, and cholesterol, which are absorbed in the upper segment of the jejunum into the portal vein. Within the smooth endoplasmic reticulum of intestinal epithelial cells, long-chain fatty acids and 2-monoglycerides can be resynthesized into triglycerides. These triglycerides combine with apolipoproteins, phospholipids, and cholesterol to form chylomicrons, which enter systemic circulation via lymphatic vessels. True chylous ascites may occur due to ruptured lymphatic vessels in the small intestine. In addition to the small intestine, triglycerides are also synthesized in the liver and adipose tissue, with the liver playing a particularly crucial role.
Monoglycerides, fatty acids, and cholesterol entering the liver may undergo oxidation to generate energy or participate in gluconeogenesis, where excess lipids are converted into glycogen and glucose. Lipid malabsorption, increased hepatic synthesis of triglycerides, and reduced export of triglycerides from hepatocytes are key pathophysiological processes underlying the development of fatty liver disease.
Proteins
Proteins are hydrolyzed by gastric and pancreatic proteases, yielding one-third as amino acids and two-thirds as oligopeptides. Oligopeptides are further hydrolyzed by oligopeptidases on the brush border of intestinal epithelial cells into amino acids. These amino acids are then actively transported into cells along with Na+ via amino acid carrier proteins. The γ-glutamyl cycle facilitates the transport of amino acids into intestinal cells. The digested and absorbed amino acids (exogenous) mix with amino acids released by the breakdown of body proteins (endogenous), forming a metabolic amino acid pool distributed throughout the body. This pool primarily functions in the synthesis of proteins and peptides.
The liver not only synthesizes proteins required for its own function but also produces albumin, some globulins, fibrinogen, prothrombin, and clotting factors. Amino acid catabolism primarily involves deamination, the metabolism of α-keto acids, and the detoxification of ammonia into urea, primarily occurring in the liver. Certain undigested proteins exhibit antigenicity, potentially triggering allergic reactions or exacerbating intestinal mucosal immune disorders. Intestinal bacteria can act on undigested proteins, resulting in putrefaction, with many of the resultant products being harmful to the human body.
Severe liver damage significantly reduces albumin synthesis, which is a major mechanism leading to edema or ascites. Hepatic injury also causes a marked elevation in serum alanine transaminase (ALT) levels. Diminished capacity for ammonia clearance results in hyperammonemia, a key mechanism in the development of hepatic encephalopathy.
Hepatic Metabolism and Detoxification
The liver is an essential organ that plays a central role in metabolism and detoxification. Its functions involve four main types of biochemical reactions:
- Oxidation: For example, ethanol is oxidized into acetaldehyde, acetic acid, carbon dioxide, and water, a process referred to as oxidative detoxification.
- Reduction: An example includes the reduction of trichloroacetaldehyde into trichloroethanol, thereby neutralizing its sedative effect.
- Hydrolysis: Various drugs or toxins are hydrolyzed by hepatic hydrolase enzymes.
- Conjugation: The most critical transformation pathway in the liver involves the conjugation of drugs or toxins with substances such as glucuronic acid, which facilitates their excretion through bile and urine.
All biochemical reactions in the liver require the involvement of enzymatic systems within hepatocytes. Therefore, in cases of severe liver disease or portal hypertension with portosystemic shunting, careful consideration of drug selection and dosage is necessary to minimize the hepatic burden and the potential for adverse drug reactions.
Coordinated Biliary Motility
The production of bile by hepatocytes begins at bile canaliculi, whose secretion relies on bile salt-dependent and bile salt-independent transport systems on the apical membranes of hepatocytes. Bile is transported through bile canaliculi, which are approximately 1 μm in diameter, in a direction opposite to that of portal venous blood flow. It subsequently flows into the canal of Hering and passes through interlobular bile ducts, left and right hepatic ducts, and the common hepatic duct. The common hepatic duct merges with the cystic duct to form the common bile duct, which then empties into the duodenum. Epithelial cells of the bile ducts secrete large amounts of water and bicarbonate, which join the bile flow. Together with the gallbladder, these structures form a system for bile collection, storage, and transportation. The sphincter of Oddi, located between the terminal bile/pancreatic duct and the duodenal papilla, regulates gallbladder filling, bile and pancreatic juice release into the duodenum, the prevention of duodenal content reflux, and maintenance of normal biliopancreatic pressure.
The liver continuously secretes bile, but bile is released into the duodenum primarily during food digestion. During inter-digestive periods (fasting), the sphincter of Oddi contracts, closing the distal end of the bile duct, increasing intraluminal pressure, and causing the relaxation of the gallbladder wall. Bile passively flows into the gallbladder, where much of its water and electrolytes are reabsorbed, concentrating the bile. The gallbladder typically holds 20–50 mL of bile. After food intake, cholecystokinin secreted by the small intestine induces gallbladder contraction and relaxation of the sphincter of Oddi, allowing bile to be released into the duodenum. Gallstones moving within the biliary tract can produce a wide range of clinical manifestations. Given the irreplaceability of the common bile duct, minimally invasive treatment strategies are often recommended for its diseases.
Physiological Mechanism of Pancreatic Enzyme Synthesis, Activation, and Self-Digestion Prevention
Under physiological conditions, various inactive pancreatic enzyme precursors (e.g., trypsinogen, amylase precursor, lipase precursor, elastase precursor, phospholipase precursor, and chymotrypsinogen) and lysosomal hydrolases are synthesized in the rough endoplasmic reticulum of acinar cells and then transported to the Golgi apparatus. Lysosomal hydrolases, after undergoing glycosylation and phosphorylation, are transported to lysosomes by specific binding to mannose-6-phosphate receptors. In contrast, trypsinogen does not bind to mannose-6-phosphate receptors. Through these two distinct pathways, digestive enzyme precursors and lysosomal hydrolases synthesized in the rough endoplasmic reticulum are ultimately sorted into different secretory vesicles, forming digestive enzyme granules and lysosomes, respectively.
In response to various physiological stimuli, an increase in intracellular calcium ion concentrations facilitates the release of digestive enzyme granules from acinar cells. These enzyme precursors pass through the pancreatic duct and duodenal papilla into the duodenum, where they are activated by enterokinase to perform their digestive functions. Trypsin, which can activate multiple other pancreatic enzymes, represents the most critical component in the cascade activation of pancreatic enzymes. Under physiological conditions, a small amount of trypsinogen secreted by acinar cells may become activated within the pancreas. However, enzyme-specific inhibitors produced by pancreatic stromal cells (e.g., α1-antitrypsin, α2-macroglobulin) quickly deactivate prematurely activated trypsin within the pancreas, preventing self-digestion from occurring.
Endoscopic Diagnosis
Gastroscopy and Colonoscopy
Gastroscopy is the most commonly used and accurate method for diagnosing diseases of the esophagus, stomach, and duodenum, while colonoscopy is primarily used to examine pathological changes from the anus to the ileocecal valve. Endoscopic examination not only allows for direct visualization of mucosal lesions but also facilitates biopsy sampling. With the continuous improvement of endoscopic equipment, technologies such as chromoendoscopy, magnification endoscopy, narrow-band imaging, and confocal endoscopy have enhanced the ability to detect early-stage tumors effectively.
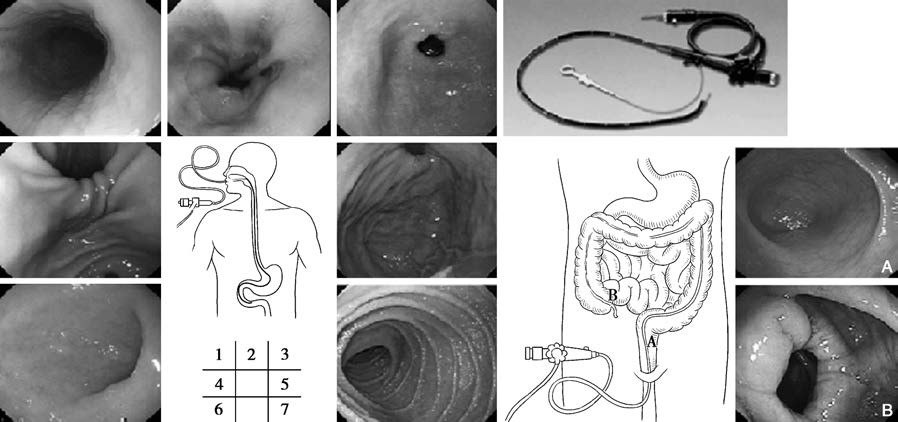
Figure 1 Gastroscopy and colonoscopy
1, Esophagus
2, Gastroesophageal junction (Z-line)
3, Gastric antrum and pylorus
4, Angular incisure
5, Fundus of the stomach
6, Duodenal bulb
7, Descending part of the duodenum
A. Rectum
B. Ileocecal region
During gastroscopy or colonoscopy, intravenous administration of short-acting sedatives and anesthetics under close monitoring can reduce patient discomfort, including nausea, vomiting, and agitation, resulting in better cooperation during the procedure. These measures also help reduce oral secretions and gastrointestinal motility, facilitating the observation and biopsy of lesions. Patients generally do not experience discomfort after awakening from the procedure.
Gastroscopy and colonoscopy also enable therapeutic interventions, such as hemostasis for various bleeding lesions, the removal of foreign bodies in the stomach, and the use of snares, electrocautery, or argon plasma coagulation to excise small or pedunculated polyps and other benign tumors. Larger benign tumors and early cancers may be treated with endoscopic mucosal resection or submucosal dissection as appropriate. Endoscopic therapy has significantly reduced the need for open surgery, offering more precise and less invasive treatment, while reducing complications, medical costs, and hospital stays.
Capsule Endoscopy
Capsule endoscopy comprises a capsule, a signal-receiving system, and a workstation. During the examination, the patient swallows a capsule containing a miniature camera, which captures continuous images of the gastrointestinal lumen at a rate of two frames per second as it moves through the gastrointestinal tract. The captured images are transmitted to the signal-receiving system and subsequently reviewed on the workstation. Capsule endoscopy provides dynamic, high-definition visualization of small bowel lesions, overcoming the limitations of previous methods for small intestine examination. It is a safe, painless, and first-line diagnostic tool for suspected small bowel diseases.
Enteroscopy
Unlike capsule endoscopy, enteroscopy features suction and air insufflation capabilities, enabling clearer visualization of lesions. It also allows for biopsy and therapeutic interventions. However, enteroscopy rarely allows for the observation of the entire small intestine and has a lower positive detection rate of small bowel lesions compared to capsule endoscopy. Furthermore, the procedure is time-consuming and often uncomfortable for patients. It is typically used after capsule endoscopy has identified small bowel lesions that require further direct examination, biopsy, or endoscopic treatment.
Endoscopic Retrograde Cholangiopancreatography (ERCP)
ERCP involves the insertion of a contrast catheter into the common bile duct or pancreatic duct via the duodenal papilla under direct visualization with a duodenoscope. The injection of contrast agent in a retrograde manner enables X-ray imaging of the biliary and pancreatic ducts. While originally a diagnostic method, ERCP is now predominantly used for therapeutic purposes in biliopancreatic disease management. Therapeutic ERCP procedures include endoscopic sphincterotomy, bile duct stone removal, stricture dilation, stent placement, and nasobiliary drainage. Its minimally invasive, effective, and repeatable characteristics have reduced the need for conventional surgical approaches.
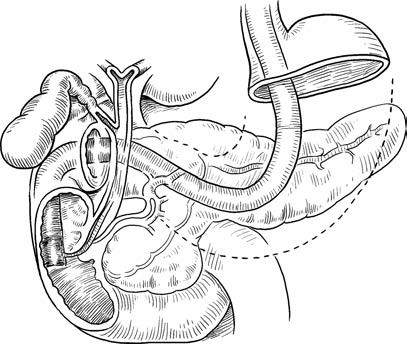
Figure 2 ERCP
Endoscopic Ultrasound (EUS)
EUS integrates a high-frequency miniature ultrasound probe attached to the tip of the endoscope or inserted through a working channel. This technique enables simultaneous direct visualization of the luminal pathology and real-time ultrasound scanning to assess the affected layers of the gastrointestinal wall and surrounding organs. Compared to surface ultrasonography, EUS reduces or eliminates the interference of bone, fat, and air-filled structures, shortens the sound path, and decreases sound attenuation. These features allow for clearer echographic imaging. EUS enables fine-needle aspiration biopsy, interventional treatments for tumors, cyst drainage, and celiac plexus block procedures for pain management.
Laboratory Examinations
Diagnosis of Hepatitis B Virus Infection
The diagnosis of hepatitis B virus (HBV) infection includes the detection of five serological immune markers (HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb), serum viral tests (HBV-DNA quantification, HBV genotyping, and detection of HBV drug-resistant mutations), and tissue virology testing (HBsAg, HBcAg, and HBV-DNA in liver tissue).
The five commonly used serological immune markers of HBV are used to determine whether a patient is infected with HBV, while HBV-DNA quantification reflects the level of viral replication. These tests are frequently used to guide antiviral treatment decisions and evaluate therapeutic efficacy.
Helicobacter Pylori Testing
Helicobacter pylori (Hp) testing plays a significant role in the diagnosis and treatment of diseases such as precancerous gastric conditions, peptic ulcers, and gastrointestinal mucosa-associated lymphomas.
Non-Invasive Methods
Common tests include the 13C- or 14C-urea breath test (Hp-UBT). This test does not require endoscopy, is well-tolerated by patients, and has high accuracy, making it one of the primary methods for detecting Hp. It has been widely adopted in hospitals. However, Hp-UBT has certain limitations, as its results can be affected by antibiotics, bismuth compounds, and acid-suppressing drugs. Another method involves the detection of Hp antigens in stool samples using monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA), which is simple, convenient, and comparable to Hp-UBT in terms of sensitivity and accuracy. However, stool antigen testing is less commonly used in clinical practice compared to the breath test.
Invasive Methods
These primarily include rapid urease tests, histological staining and microscopic examination of gastric mucosal tissue (e.g., Warthin-Starry silver staining), and bacterial cultures. Gastric mucosal sampling for bacterial culture is generally not used for routine clinical diagnosis and is primarily reserved for research purposes.
Assessment of Liver Function
Synthetic Function of the Liver
Serum Albumin
Albumin is synthesized exclusively by hepatocytes. A significant decline in serum albumin levels indicates impaired hepatic synthetic function. In stable conditions, some patients may still have albumin levels within the normal range, but events such as bleeding, infections, or surgeries can lead to a pronounced drop, often making recovery to normal levels challenging.
Plasma Coagulation Factors
Most coagulation factors are synthesized in the liver and have a much shorter half-life than albumin, particularly the vitamin K-dependent factors (II, VII, IX, X). In the early stages of liver dysfunction, albumin levels may remain normal, while vitamin K-dependent coagulation factors markedly decline. The prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) are commonly used parameters.
Cholesterol
Approximately 70% of endogenous cholesterol is synthesized in the liver. Hepatic dysfunction can lead to a reduction in blood cholesterol levels.
Hepatocyte Damage
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are present in the cytoplasm of hepatocytes. When hepatocyte membranes rupture, ALT and AST levels rise significantly, making them important indicators of liver cell damage. However, as AST is also present in skeletal muscle, kidney, and cardiac tissues, elevations of AST alone may not necessarily indicate liver damage. Within hepatocytes, AST is mainly located in mitochondria. A significant increase in AST alongside elevated ALT suggests severe liver cell damage. In cases of severe hepatitis, a decrease in transaminase levels accompanied by an increase in bilirubin reflects widespread hepatocellular necrosis, with a mortality rate as high as 90%. ALT and AST levels are typically mildly to moderately elevated in chronic liver diseases. In liver cirrhosis, characterized pathologically by fibrosis and hepatocyte atrophy, ALT and AST levels are often normal in many patients.
Bilirubin Metabolism
Bilirubin is a product of the breakdown of aged red blood cells by the mononuclear phagocyte system in the liver, spleen, and bone marrow. Total bilirubin (TB) includes unconjugated bilirubin (UCB) and conjugated bilirubin (CB). Unconjugated bilirubin, a metabolic product of hemoglobin, is taken up by hepatocytes and conjugated with glucuronic acid to form water-soluble conjugated bilirubin, which is excreted through the biliary tract. Jaundice can occur if any step in this process is disrupted. Serum bilirubin testing can detect jaundice that is not yet clinically apparent and often reflects liver cell damage or bile stasis. A positive urinary bilirubin test suggests elevated conjugated bilirubin levels in the blood, while increased urinary urobilinogen levels indicate an inability of the liver to process urobilinogen reabsorbed from the intestines.
The above liver function indicators do not entirely correspond to liver health. Therefore, liver function assessment should integrate clinical symptoms, physical examination findings, imaging results, and pathological data. Upon confirming liver damage and functional decline, identifying the underlying cause is important. The Child-Pugh score is commonly used to grade the degree of liver dysfunction for clinical decision-making. As liver function grading may fluctuate with disease progression, its application requires flexibility.
Analysis of Exocrine Pancreatic Function
This involves testing various digestive enzymes secreted by the pancreas. Functional tests are categorized into direct and indirect methods. Direct tests evaluate pancreatic secretions after the intravenous injection of secretagogues or combinations of secretagogues. Indirect tests assess the status of exocrine pancreatic function by measuring digestive enzyme breakdown products after stimulating pancreatic secretion with a test meal.
Autoimmune Disease
This includes the detection of antibodies related to autoimmune gastritis, such as parietal cell antibodies, intrinsic factor antibodies, and pepsinogen I/II, along with various other autoantibodies.
Microbiological Testing
This encompasses the detection and culture of microorganisms in blood, feces, urine, and peritoneal fluid, as well as the analysis of antibodies produced in response to microbial infections.
Tumor Markers for Digestive System Malignancies
Common tumor markers include alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA19-9, and CA72-4, among others.
Imaging Diagnosis
Ultrasonography
Ultrasonography is widely used in clinical practice to evaluate abnormalities in solid digestive system organs, bile ducts, and intra-abdominal structures due to its non-invasive nature, lack of radiation, cost-effectiveness, convenience, speed, and ability to assess hemodynamic parameters. However, its effectiveness is limited when examining tissues or organs obscured by gas or bone, and findings are highly dependent on the operator's skill and experience.
CT (Computed Tomography)
Contrast-enhanced CT scanning is an essential diagnostic tool for small lesions, isodense lesions, lesions requiring precise localization and characterization, and vascular abnormalities in digestive system organs. Advances in CT imaging speed, resolution, improved post-processing software, more efficient image interpretation, and gradually reduced costs have enhanced its role in diagnosing abdominal diseases. However, caution or avoidance is advised during its use in patients with impaired liver or kidney function.
MRI (Magnetic Resonance Imaging)
MRI is valuable for detecting small lesions and providing qualitative diagnostic information, particularly in differentiating the histological origin of hepatic and hepatic hilar lesions, as well as in diagnosing biliary and pancreatic conditions. Magnetic resonance cholangiopancreatography (MRCP), a non-invasive imaging technique based on water visualization principles, offers a clear depiction of the bile duct and pancreatic duct lumens without requiring contrast agent administration. MRCP is particularly important in evaluating biliary and pancreatic diseases.