As the gateway to the respiratory tract, the nasal cavity and paranasal sinuses play a critical role in maintaining normal physiology, ensuring the stability of the local microenvironment, and defending against pathogenic microorganisms. The nasal cavity and sinuses serve not only as a mechanical barrier but also as an immune barrier.
Respiratory Function
Nasal Resistance and Its Physiological Significance
Nasal resistance is essential for maintaining normal nasal ventilation. It is primarily formed by various structures in the nasal valve area, which is the narrowest region of the nasal cavity. The nasal valve area includes the anterior-inferior edge of the nasal septal cartilage, the anterior edge of the lateral nasal cartilage, and the piriform aperture at the entrance of the nasal cavity. The bilateral inferior nasal conchae also contribute to the formation of nasal resistance. The presence of nasal resistance divides the airflow entering the nasal cavity into two components: laminar flow and turbulent flow. The laminar flow, which constitutes the majority of the nasal airflow, follows an arched upward and posterior path that spreads at the posterior nasal aperture. This portion of the airflow is primarily responsible for pulmonary gas exchange. Laminar flow contacts a larger surface area of the nasal cavity, optimizing the warming and humidification of incoming air. Turbulent flow, on the other hand, forms irregular vortices behind the nasal vestibule and accounts for a smaller portion of airflow. It promotes mixing of the inhaled air and enhances interaction with the nasal mucosa, facilitating the deposition of dust and particulate matter onto the nasal mucosal surface. Nasal resistance accounts for approximately 40% to 50% of the total airway resistance. The presence of normal nasal resistance helps create negative pressure in the thoracic cavity during inhalation, allowing the alveoli to expand and increasing the area available for gas exchange. Additionally, it prolongs the retention time of air in the alveoli during exhalation, allowing sufficient time for gas exchange.
Nasal Cycle
Nasal resistance undergoes rhythmic and alternating variations between the two sides of the nasal cavity during day and night. This phenomenon is influenced by the periodic contraction and expansion of the capacitance vessels within the mucosa of the bilateral inferior nasal conchae. These cycles typically occur every 2 to 7 hours and are referred to as the physiological turbinal cycle or the nasal cycle, regulated by the hypothalamus. When nasal airflow and turbulence increase, mucociliary clearance is enhanced, leading to reduced resistance and better nasal patency. Conversely, when resistance increases, nasal airflow becomes more restricted. Another function of the nasal cycle is to encourage body movement, particularly turning during sleep, which aids in relieving fatigue.
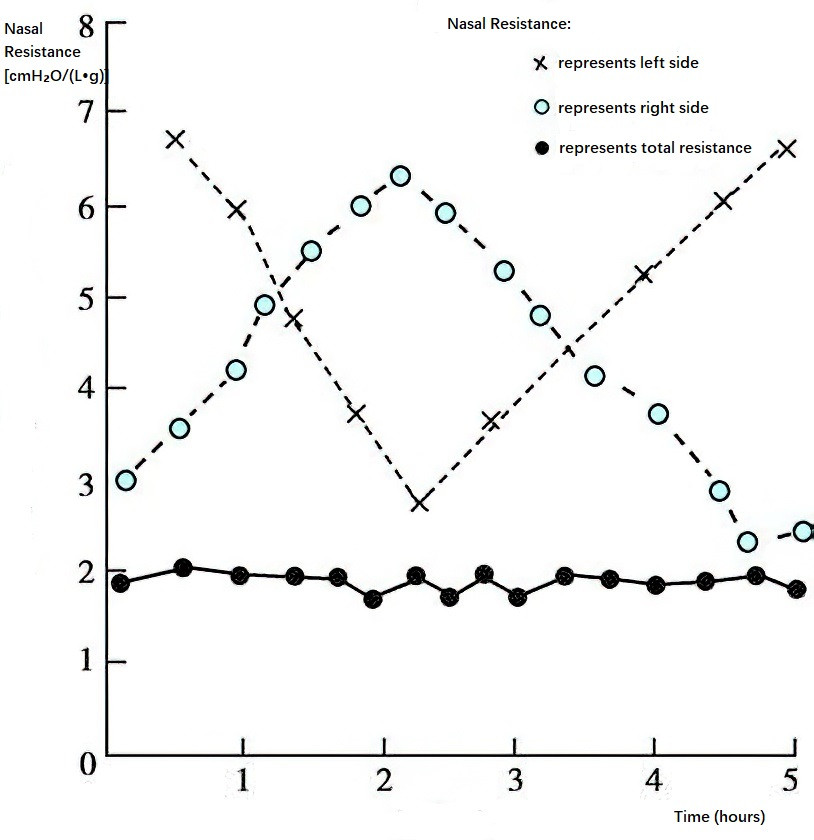
Figure 1 Schematic diagram of nasal cycle
Warming and Humidification
Air entering the nasal cavity is rapidly warmed to near-body temperature to protect the mucosa of the lower respiratory tract. The temperature of the inferior nasal conchae is typically maintained between 33°C and 35°C. This function relies on the nasal mucosa and its rich blood supply, including abundant arterial blood flow at arteriovenous anastomoses and the spongy venous sinuses in the lamina propria of the inferior nasal conchae. These blood vessels dilate or contract in response to ambient temperature changes, adjusting the nasal cavity's volume and modulating airflow speed to regulate the temperature of inhaled air. The nasal mucosa contains numerous glands (e.g., mucus and serous glands in the respiratory epithelial layer) and secretory epithelial cells (e.g., goblet cells). These glands and cells, along with capillary exudates, constitute the main sources of nasal secretions. The nasal cavity produces approximately 1,000 mL of secretions daily, with about 70% used for humidification. A small portion flows into the pharynx, while tears entering the nasal cavity through the nasolacrimal duct also assist in humidification. Before reaching the lungs, the humidity of inhaled air is increased by more than 80%. Warmed and humidified airflow facilitates gas exchange in the alveoli and supports the normal ciliary motion of the respiratory mucosa.
Filtration and Cleaning
The nasal vestibule's nasal hairs act as a barrier and filter for larger dust particles and microorganisms in the air. Smaller particles of dust settle in the turbulent airflow or are deposited in the mucus blanket by laminar flow. Soluble particles dissolve in the mucus, while insoluble ones are transported to the nasopharynx by ciliary movement and then swallowed or expelled. The nasal cavity can filter 95% of inhalable particles larger than 15 μm in diameter, though its filtration for smaller particles such as pollen and dust is weaker. Breathing through the nose rather than the mouth effectively removes harmful inhaled gases, such as smoke. The sneeze reflex aids in expelling dust and small foreign objects that enter the nasal cavity. The cleaning function of the nasal cavity primarily relies on the mucociliary system on the nasal mucosal surface.
Mucociliary Clearance (MCC)
Most of the nasal and sinonasal mucosa is lined with pseudostratified ciliated columnar epithelium. Each ciliated epithelial cell contains about 250–300 cilia, each measuring 5–10 μm in length and approximately 0.3 μm in diameter. The cilia beat toward the nasopharynx at a frequency of about 1,000 times per minute. The cilia are covered by a mucus blanket, whose main components include inorganic salts, glycosaminoglycans, mucin, lysozyme, and 95% water. This mucus blanket moves at a rate of 5 mm per minute, creating a front-to-back mucus wave that plays a crucial role in maintaining nasal cleanliness. Each ciliated columnar cell also contains 300–400 microvilli, which increase the epithelial cell surface area, facilitate water and substance exchange, and help retain moisture to sustain ciliary motion.
The mucus blanket is about 10–15 μm thick and comprises two layers: a viscous mucus layer (gel layer) on the top, originating from the mucus glands, and a thinner serous layer (sol layer) on the bottom. The cilia can move freely within the sol layer without entering the more viscous gel layer. In the nasal cavity, ciliary motion is directed from the anterior to the posterior toward the posterior nasal aperture, while in the sinuses, ciliary motion moves from the sinus wall periphery toward the natural sinus ostia.
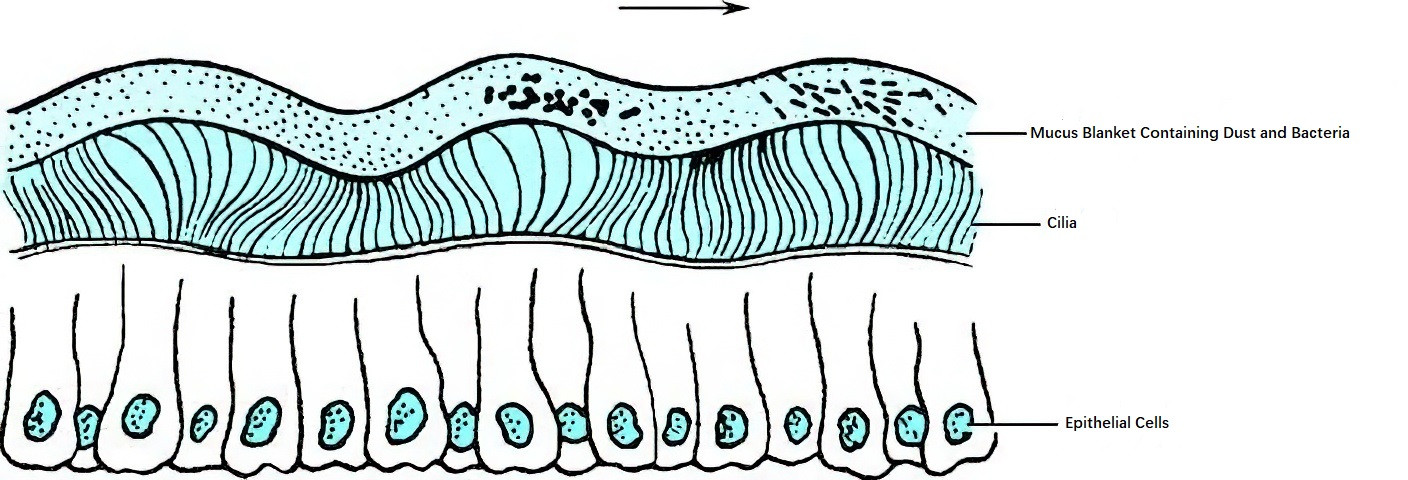
Figure 2 Motion pattern of nasal mucosal cilia and mucus blanket (the arrow indicates the direction of motion)
Ciliary motility is an essential mechanism for maintaining normal nasal physiological function. Mucociliary clearance relies on the interaction between nasal mucus and ciliary motion to ensure cleanliness throughout the upper and lower respiratory tracts. Factors such as the number, structure, and coordinated movement of the cilia, as well as the biochemical, physical, and chemical properties of the mucus, are equally important. Nasal mucus is slightly acidic, with a pH of 5.6–6.5, and it traps finer dust particles and bacteria within the mucus blanket. These particles are transported to the nasopharynx via epithelial ciliary motion, forming an important first line of defense.
Nasal mucus contains lysozyme, which inhibits and dissolves bacteria, while leukocyte phagocytosis further enhances nasal defense. Together with the immune system, the mucociliary clearance system maintains a sterile environment in the sinuses under physiological conditions. In pathological conditions, such as congenital ciliary dyskinesia, mucociliary clearance may become impaired to varying degrees, predisposing individuals to upper respiratory tract infections, including sinusitis.
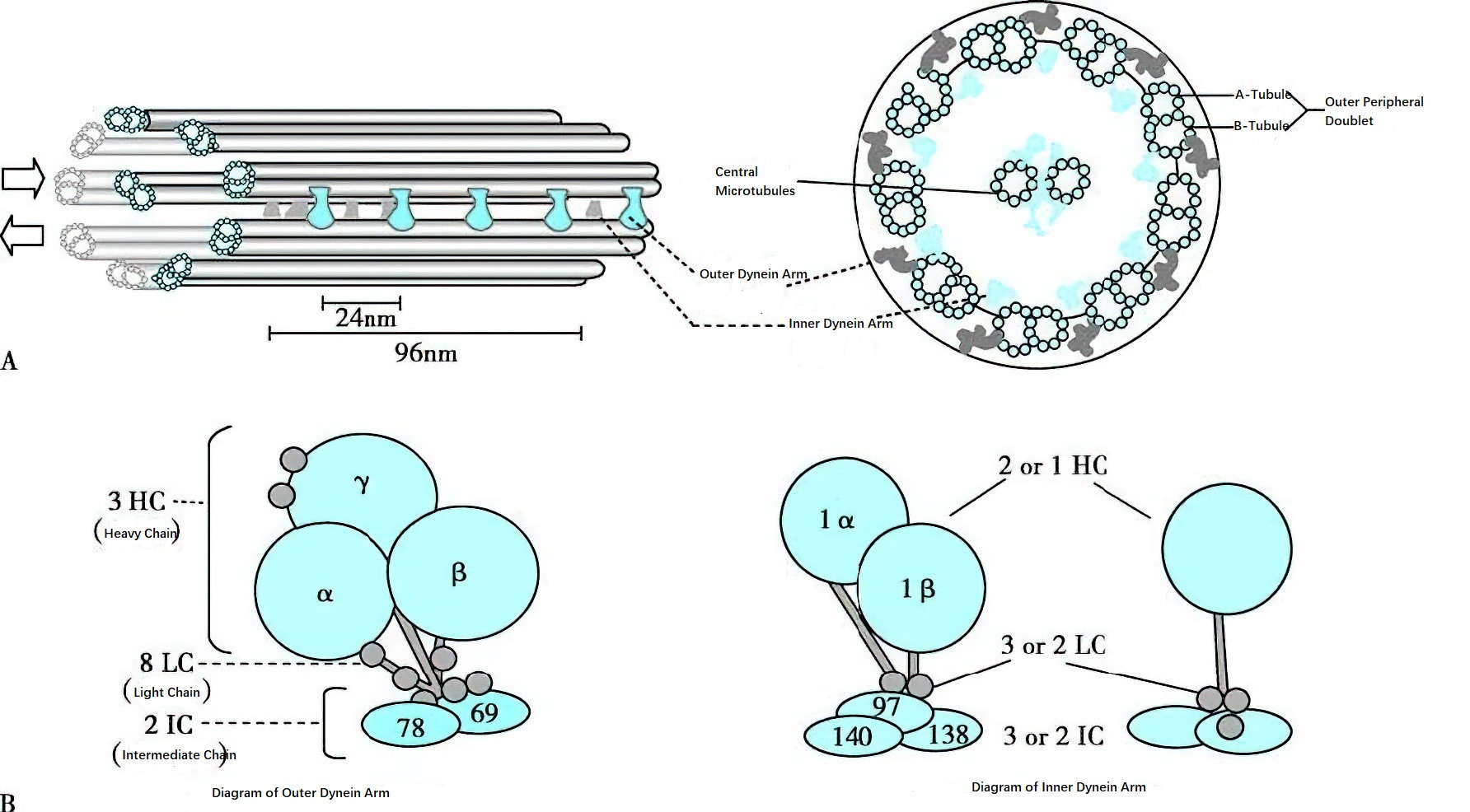
Figure 3 Schematic diagram of ciliary ultrastructure
A. Dynein arms with ATPase drive the sliding of each pair of microtubules in the direction indicated by the arrow, resulting in the ciliary motion. Dynein arms are regularly distributed along the microtubules, with the outer dynein arm spaced at 24 nm intervals and the inner dynein arm spaced at 96 nm intervals.
B. Dynein arms are protein complexes composed of several subunits.
Changes in the rheology of surface fluids on the mucosa represent another factor affecting mucociliary clearance. A classic example is cystic fibrosis, often seen in Caucasian populations, where sinusitis is often one of the first presenting conditions. This disorder is characterized by elevated sweat chloride levels above 60 mmol/L. Its fundamental physiological defect involves abnormal epithelial transport of water and electrolytes, leading to insufficient hydration of mucus secretions. This results in mucus obstruction and subsequent inflammation. Defective mucosal surface fluid rheology disrupts the mucociliary clearance system, facilitating bacterial colonization and local inflammation. Changes in nasal secretions, including subtle alterations in the quality and quantity of the peri-ciliary fluid, have a greater impact on the dysfunction of mucociliary clearance in sinusitis than structural abnormalities of the cilia themselves.
Olfactory Function
Components of the Olfactory System
The olfactory system consists primarily of the olfactory epithelium, the olfactory bulb, and the olfactory cortex. The olfactory sensory cells in the olfactory epithelium extend peripheral processes toward the mucosal surface. The tips of these processes swell to form olfactory knobs, which contain cilia that increase the surface area of the olfactory region. The central processes lack myelin sheaths and converge to form olfactory filaments, which traverse the cribriform plate to terminate in the olfactory bulb.
Axons from the olfactory bulb form the olfactory tract, which lies within the olfactory sulcus of the frontal lobe. The tract extends posteriorly and terminates in the olfactory cortex. The olfactory sensory neurons contain olfactory receptors on their surfaces.
Mechanism of Olfaction
Odors that are perceived in daily life arise from a mixture of molecules in the air, which stimulate the olfactory receptors on the nasal mucosa. These molecules, referred to as odorants or odor molecules, initiate the olfactory response only after binding to olfactory receptors on the olfactory mucosa. Binding of the odorant to its receptor induces a nerve impulse that is transmitted via the olfactory nerve to the olfactory bulb. Within the olfactory bulb, olfactory information undergoes encoding and processing before being transmitted to the olfactory cortex, where it is decoded into distinct smells. During normal breathing, only 5–10% of the inspired air reaches the olfactory region. With forceful inhalation, accelerated airflow directs more air toward the olfactory region. Short and forceful breathing enhances odor recognition.
Neural Control of Olfaction
The olfactory nerve primarily governs the sense of smell. Additionally, cranial nerves V (trigeminal), VII (facial), IX (glossopharyngeal), and X (vagus) play a supportive role in olfactory function.
Immune Function
The normal physiological functions of the nasal cavity and sinuses depend on the immune system, primarily involving innate immunity and adaptive (or acquired) immunity.
Innate Immune Response
Innate immunity of the nasal mucosa, also referred to as nonspecific immunity, mainly involves nasal epithelial cells, innate immune cells, and innate immune molecules. The epithelium, which is primarily composed of basal cells, ciliated cells, and goblet cells, forms the first barrier of the nasal mucosa through tight intercellular junctions and mucociliary clearance. Additionally, epithelial cells recognize pathogen-associated proteins via pattern recognition receptors (PRRs) and secrete anti-infective factors such as type I and type III interferons, thereby initiating innate immune responses.
Innate immune cells within the mucosa include dendritic cells, macrophages, neutrophils, eosinophils, monocytes, mast cells, innate T cells, and innate lymphoid cells. These cells also sense microbial antigens through PRRs and activate innate immune responses. Findings such as the extracellular traps formed by neutrophils and eosinophils (NETs and EETs) and the regulatory mechanisms of type 2 innate lymphoid cells have further expanded the scope of nasal mucosal innate immunity research.
The mucus layer on the nasal mucosal surface contains abundant innate immune substances, including lysozyme (which attacks the cell wall of gram-positive bacteria), lactoferrin (which inhibits bacterial growth), oligosaccharides (which bind to bacteria), and localized immunoglobulin (which neutralizes microbial antigens). The host response of epithelial cells, innate immune cells, and innate immune molecules to invading pathogens, as well as the molecular events involved, constitute the most significant innate immune mechanisms of the nasal mucosa.
Adaptive Immune Response
Epithelial cells and innate immune cells recognize microbial ligands present on bacterial or viral surfaces through PRRs, such as Toll-like receptors. This activation induces the expression of costimulatory molecules and the release of a variety of inflammatory factors (e.g., IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α) and chemokines, which regulate the actions of adaptive immune cells such as helper T cells, cytotoxic T cells, and B cells. Epithelial-derived type 2 cytokines (e.g., TSLP, IL-25, IL-33) also promote the infiltration and activation of eosinophils, type 2 innate lymphoid cells, and Th2 cells, making these cytokines key upstream factors in type 2 immune responses in the nasal mucosa.
This indicates that pattern recognition receptors and certain epithelial-derived factors serve as bridges between innate immunity and adaptive immunity. Additionally, innate immune cells such as dendritic cells and macrophages present antigens from pathogenic proteins to activate local helper T cells, resulting in a spectrum of immune responses (e.g., Th1, Th2, Th17), which form the foundation of antigen-specific immune responses in the nasal mucosa.
Lymphocytes residing in the mucosa-associated lymphoid tissue of the nasal submucosa exhibit mucosal homing properties, restricting adaptive immune responses to the nasal mucosa. Memory B cells and plasma cells, which produce antibodies, function as key effector cells and molecules in adaptive immune responses targeting specific microbial or host antigens. Consequently, the nasal mucosal immune system maintains homeostasis through the interplay of innate and adaptive immunity.
As research on nasal mucosal immunity progresses, the pathogenesis of various diseases is being elucidated. For example, nasal mucosal immunity has been shown to involve interactions between peripheral immune cells and the central nervous system (neuroimmune interactions). This discovery is expected to deepen understanding of airway inflammation mechanisms and facilitate translational applications.
Function in Vocal Resonance
The three-dimensional structure of the nasal cavity and sinuses contributes to resonance, producing sounds that are rich and unique to each individual. Nasal resonance is part of speech formation, and its degree directly influences the quality of speech. Nasal obstruction leads to a condition known as hyponasal resonance, whereas cleft palate results in hypernasal resonance.
Reflex Functions of the Nose
The nasal cavity is richly innervated, and mechanical or chemical stimuli to the nasal mucosa can elicit a wide range of respiratory and circulatory system responses. The intensity of such responses depends on the severity of the stimulus.
Sneeze Reflex
The afferent pathway of the sneeze reflex is mediated by the trigeminal nerve. When foreign substances enter the nasal cavity and stimulate the trigeminal nerve endings in the nasal mucosa, a series of reflex actions ensues. These include deep inhalation, descent of the uvula, and elevation of the tongue root. The abdominal muscles and diaphragm contract forcefully, followed by a sudden opening of the glottis, expelling air rapidly through the nose and mouth at speeds of up to 50 m/s. This mechanism serves to remove foreign particles or irritants from the nasal cavity.
Nasopulmonary Reflex
This reflex involves the trigeminal nerve endings in the nasal mucosa as the afferent pathway, with the vagus nerve fiber endings in the bronchial smooth muscle serving as the efferent pathway. The trigeminal and vagus nerve nuclei function as the central processing centers of this reflex arc. The nasopulmonary reflex is thought to play a role in the relationship between localized nasal irritation or pathological changes and the onset of bronchial conditions.
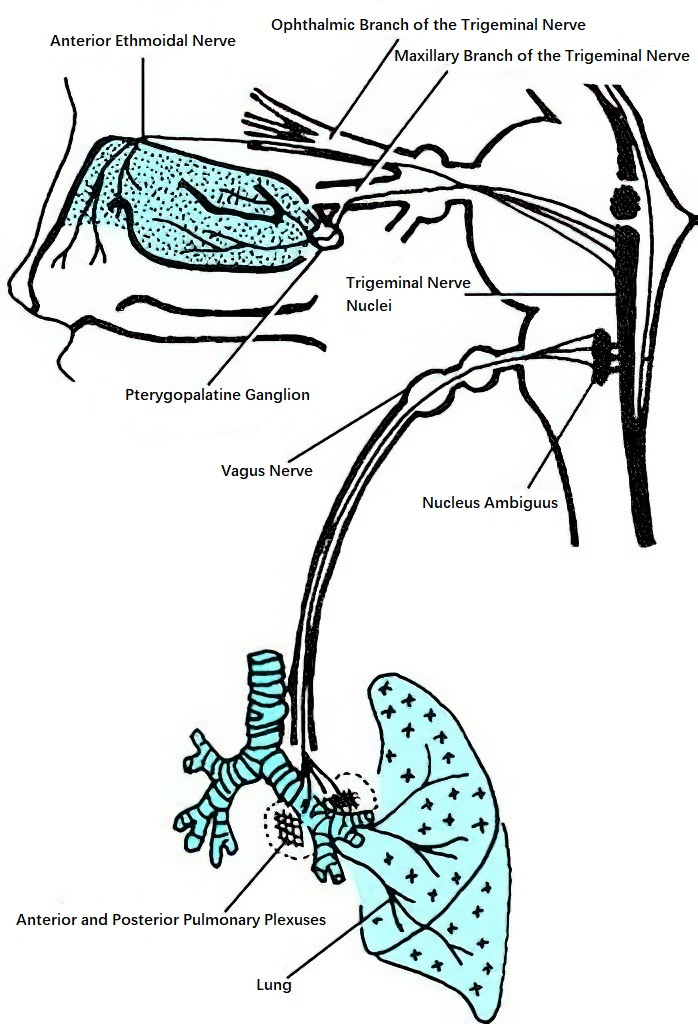
Figure 4 Schematic diagram of the nasopulmonary reflex
Nasolacrimal Reflex
Chemical or mechanical stimulation of the nasal cavity can trigger the nasolacrimal reflex, increasing tear secretion. C-class unmyelinated nerve fibers transmit the stimulus via the trigeminal nerve to the superior salivatory nucleus. The efferent fibers enter the geniculate ganglion and travel through the greater superficial petrosal nerve to the pterygopalatine ganglion. Postganglionic parasympathetic fibers then reach the lacrimal gland via the zygomatic branch, regulating tear secretion. The reflex arc of the nasolacrimal reflex originates in the sensory nerves of the nasal cavity and terminates at the target lacrimal gland, which is controlled by parasympathetic innervation. This reflex plays a crucial role in maintaining tear film homeostasis by coordinating baseline and reflexive tear secretion.