General Characteristics of Hearing
Hearing refers to the sensation provoked by sound acting on the auditory system. The frequency range detectable by the human ear lies between 20 and 20,000 Hz, with maximum sensitivity to frequencies between 1,000 and 3,000 Hz. Sound must reach a certain intensity to produce auditory sensations; the minimum sound intensity capable of eliciting hearing is referred to as the hearing threshold. The hearing threshold of the human ear varies depending on frequency, with the greatest sensitivity typically occurring at 1,000 Hz. Above the hearing threshold, the volume of sound increases as the auditory stimulus strengthens. When sound pressure reaches excessive levels, tactile sensations, pressure sensations, and pain may occur in the ear. The sound intensity that just elicits tactile or painful sensations in the ear is called the threshold of feeling or the threshold of pain. The threshold of feeling also varies with different sound frequencies. The area between the hearing threshold curve and the threshold of feeling curve represents the auditory sensory area, which encompasses the full range of frequencies and intensities capable of producing auditory sensations within the auditory system.
Pathways of Sound Transmission to the Inner Ear
Sound is transmitted to the inner ear primarily via the tympanic membrane and the ossicular chain, but it can also be conducted through the bones of the skull. The former transmission pathway is known as air conduction (AC), while the latter is referred to as bone conduction (BC). Under normal conditions, air conduction is the primary mechanism of sound transmission.
Air Conduction (AC)
Air conduction typically follows a pathway: external ear → tympanic membrane → ossicular chain → oval window → inner ear lymphatic fluid. From the perspective of auditory physiology, the external ear collects sound, the middle ear transmits it, and the inner ear performs sensory functions. Vibrations of the stapes footplate in the oval window cause waves in the perilymph of the inner ear, which, in turn, lead to reciprocal movements of the membrane of the round window. These waves in the inner ear lymphatic fluid induce vibrations in the basilar membrane, stimulating the auditory hair cells of the spiral organ (Organ of Corti). The perilymph and endolymph in the cochlea play roles in sound conduction. When the perilymph flows slowly, the wave travels from the vestibular duct to the scala tympani via the helicotrema, causing outward movement of the round window. When the flow is rapid, the wave displaces the cochlear duct and its contents toward the scala tympani.
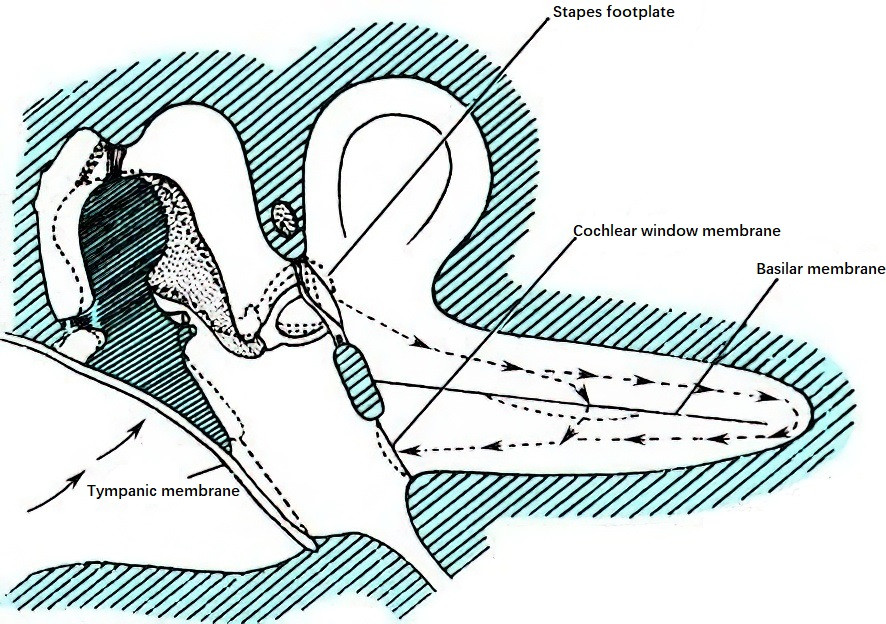
Figure 1 Pathway of sound transmission
Bone Conduction (BC)
Bone conduction refers to the direct transmission of sound waves to the perilymph of the inner ear through vibrations of the skull, which activate the spiral organ of the cochlea to produce hearing. In normal auditory function, the contribution of bone conduction to hearing is minimal and thus lacks practical significance for most purposes. However, bone conduction hearing is important for the differential diagnosis of hearing loss. When sound waves are transmitted to the cochlea through the skull, the vibrations of the cochlear walls primarily stimulate the sensory receptors in the inner ear, which can occur through two main mechanisms:
Translatory Mode of Bone Conduction
When sound waves act on the skull, the entire head, including the cochlea, vibrates as a single unit. Due to the inertia of the endolymph, there is a slight delay in the movement of the lymphatic fluid relative to the walls of the cochlea during each vibration cycle. As each cycle begins, the lymphatic fluid moves in the opposite direction of the cochlear wall, causing oscillatory displacement of the basilar membrane, which stimulates the hair cells to perceive sound. The inertia of the ossicular chain also plays a role in translatory bone conduction. Since the ossicular chain is suspended within the tympanic cavity and not tightly connected to the skull, its inertia causes its movements to lag slightly behind the cochlear bony walls during skull vibrations. This lag results in stapes footplate movements akin to those induced by air conduction. Bone conduction at low frequencies, particularly below 800 Hz, is primarily influenced by this translatory mode.
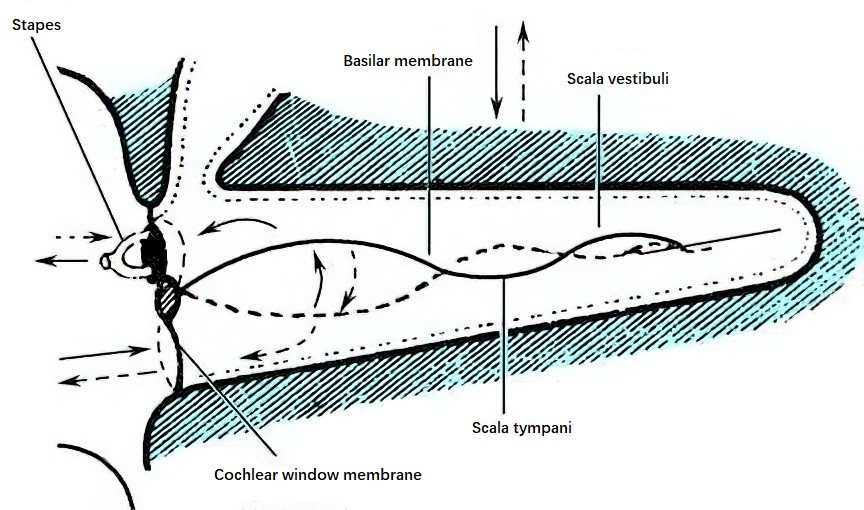
Figure 2 Diagram of translatory bone-conducted cochlear lymph flow and basilar membrane displacement following lymph motion
Compressional Mode of Bone Conduction
When sound waves transmitted through the skull vibrate the cochlear walls, the walls undergo expansion and compression based on the compression and rarefaction phases of the waves. During the compression phase, the bony walls of the labyrinth compress, driving the relatively incompressible lymphatic fluid toward the round window or the oval window. The volume ratio of the vestibular duct to the tympanic duct is 5:3, with a larger fluid capacity in the vestibular duct. Furthermore, the round window exhibits five times greater mobility than the oval window. Consequently, compression of the cochlear walls during the compression phase results in lymphatic fluid being pushed into the larger-capacity vestibular duct and then flowing toward the scala tympani, causing the round window membrane to bulge outward and the basilar membrane to shift downward. During the rarefaction phase of the sound waves, the cochlear walls expand, the lymphatic fluid returns to its original position, and the basilar membrane moves upward. The repetitive alternation between compression and rarefaction results in basilar membrane vibration, effectively stimulating the auditory hair cells to perceive sound. Bone conduction at high frequencies, particularly above 800 Hz, is mainly influenced by this compressional mode.
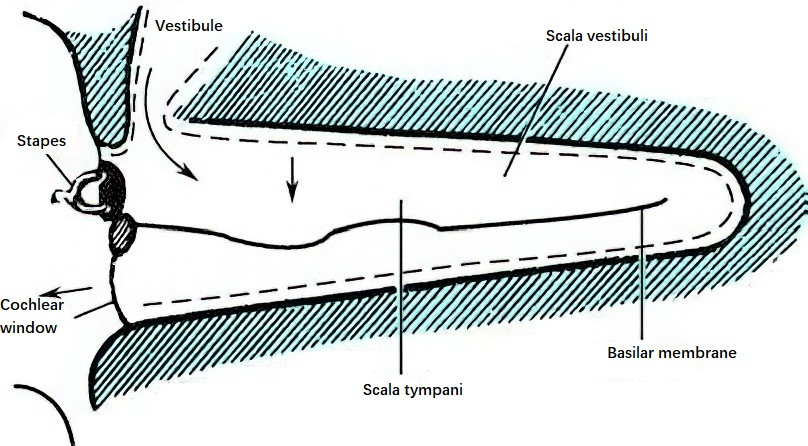
Figure 3 Diagram of compressional bone-conducted cochlear lymph flow and basilar membrane displacement toward the scala tympani
The above two mechanisms of bone conduction act synergistically, with their relative contributions varying depending on the frequency of the sound waves. Additionally, sound waves may reach the inner ear through a secondary bone-tympanic pathway, where skull vibrations caused by sound waves transmit vibrations to the external auditory canal, tympanic cavity, and surrounding air. These vibrations then follow the middle ear sound transmission system to reach the inner ear, resembling the air conduction pathway.
Physiology of the External and Middle Ear
Physiology of the External Ear
The primary function of the auricle (pinna) is to collect and transmit sound waves to the external auditory canal. Differences in the timing and intensity of sounds arriving at the two ears, combined with central nervous system processing, allow the cooperative sound-gathering function of both auricles to help discern the direction of sound sources. The external auditory canal transmits sound and amplifies it through resonance. The resonant peak of the human external auditory canal is approximately 3,500 Hz. It is estimated that sound with a frequency of 3,000 Hz can increase sound pressure near the tympanic membrane by about 15 dB, while frequencies of 2,000 Hz or 5,000 Hz can increase sound pressure by approximately 10 dB.
Physiology of the Middle Ear
The middle ear serves to transfer sound wave energy from the air in the external auditory canal to the cochlear lymphatic fluid, thereby stimulating the structures of the inner ear and enabling hearing. The process of sound transmission in the middle ear functions as an impedance matching system. When two media have the same acoustic impedance, sound energy is transmitted between them most efficiently. Conversely, the greater the difference in acoustic impedance between two media, the less efficient the transmission of sound energy. Since the acoustic impedance of water is significantly higher than that of air, only about 0.1% of the energy from airborne sound enters water, with the remaining sound energy being reflected at the water surface (resulting in a loss of approximately 30 dB). The primary function of the middle ear is to perform impedance matching, which allows the high resistance of fluid to sound wave transmission to be matched with the much lower resistance in air. This ensures the efficient transfer of sound wave vibrations from air into the lymphatic fluid of the inner ear. The middle ear achieves this impedance matching function through the sound transmission system formed by the tympanic membrane and the ossicular chain, which operates via three main mechanisms:
- The difference in surface area between the tympanic membrane and the stapes footplate.
- The lever action of the ossicular chain.
- The conical shape of the tympanic membrane, which generates a lever effect.
Physiological Functions of the Tympanic Membrane
From an acoustic perspective, the tympanic membrane functions similarly to the diaphragm of a microphone, acting as a pressure receptor with excellent frequency response and minimal distortion. The tympanic membrane typically vibrates at the same frequency as the sound wave, though the pattern of vibration varies depending on frequency. According to observations by Békésy (1941), when sound frequencies are below 2,400 Hz, the entire tympanic membrane vibrates around a rotational axis that runs tangentially along the tympanic sulcus (connecting the anterior and lateral processes of the malleus). Different parts of the tympanic membrane vibrate with varying amplitudes, with the area extending downward from the malleus handle to near the membrane's base exhibiting the largest amplitude. When sound frequencies exceed 2,400 Hz, the tympanic membrane exhibits segmented vibration, with the vibration frequency of the malleus handle being lower than that of the tympanic membrane itself.
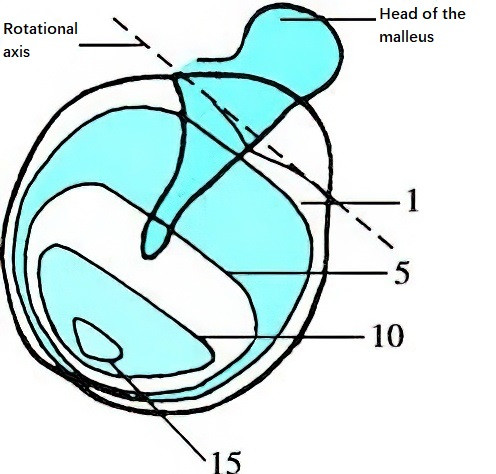
Figure 4 Vibrational amplitude of the tympanic membrane
The vibrational amplitude within each closed curve is equal, with numerical values indicating relative amplitude.
The effective vibrating area of the tympanic membrane, given its attachment to the tympanic sulcus, is approximately two-thirds of its anatomical area, or about 55 mm², which is 17 times the area of the stapes footplate (approximately 3.2 mm²). Therefore, sound pressure transmitted from the tympanic membrane to the stapes footplate can be amplified by a factor of 17. Tonndorf et al. (1970) theorized that the curvature of the conical tympanic membrane contributes a lever effect, consistent with Helmholtz's (1868) concept of the tympanic membrane's curvature inducing a pressure amplification. Studies show that the ratio of vibration amplitude between the concave surface of the tympanic membrane and the malleus handle is 2:1, indicating that the malleus handle vibrates with lower amplitude but greater strength compared to the adjacent tympanic membrane. This process effectively doubles the sound pressure. Furthermore, the curved shape of the tympanic membrane helps maintain accurate tonal quality across varying frequencies, reducing sound distortion.
Physiological Functions of the Ossicular Chain
The three ossicles form a bent lever system. Acting as a lever, the ossicular chain transmits sound wave vibrations from the tympanic membrane to the inner ear, achieving effective impedance matching. The movement axis of the ossicular chain passes forward through the malleus handle and backward through the short process of the incus. When the movement axis is treated as the fulcrum, the long process of the incus and the handle of the malleus can be regarded as the two arms of the lever, with a length ratio of 1.3:1. The masses of the ossicles on either side of the fulcrum are roughly equal. According to the principle of levers, when forces on both sides of the fulcrum are equal, the increase in force depends on the relative lengths of the lever arms. Thus, the lever action of the ossicular chain amplifies the sound pressure transferred from the malleus handle to the oval window by a factor of 1.3. In total, the sound pressure increases by approximately 1.3 × 17 = 22.1 times as the sound travels from the tympanic membrane through the ossicular chain to the stapes footplate, equivalent to a gain of about 27 dB. When the lever effect of the tympanic membrane's curvature is included, the amplification is further enhanced.
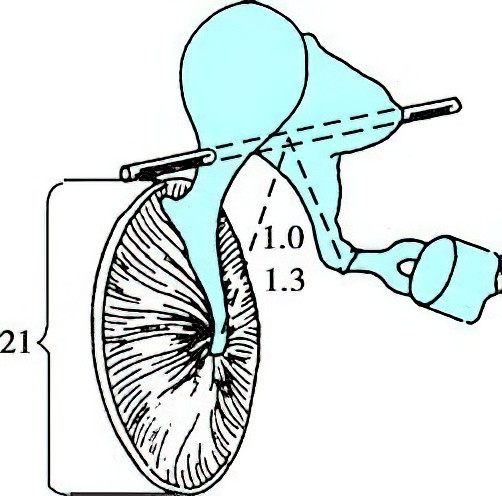
Figure 5 Schematic diagram of the tympanic membrane, ossicular chain, and its rotational axis
Numerical values indicate the area ratio between the tympanic membrane and the oval window, as well as the length ratio of the long arm to the short arm of the ossicular chain.
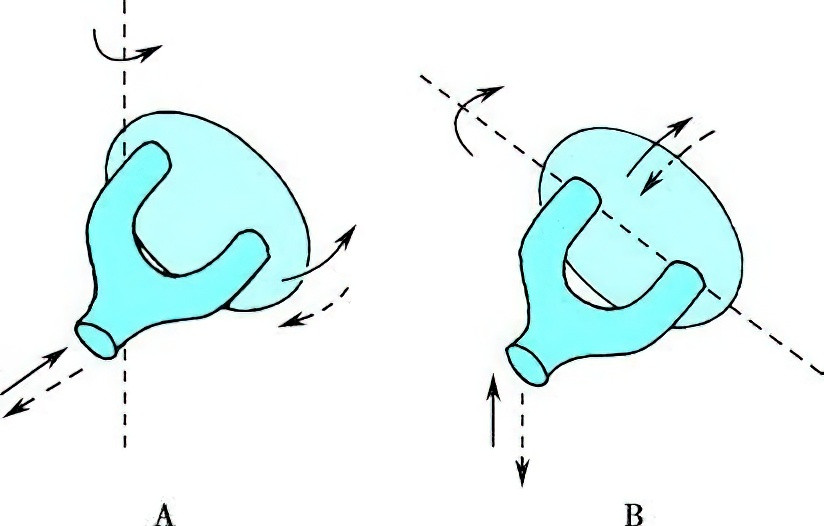
Figure 6 Rotational axis of stapes motion
A. During medium-intensity sound stimulation, the stapes footplate vibrates along a vertical axis passing through its posterior crus.
B. During high-intensity sound stimulation, the stapes footplate rotates along its anterior-posterior axis.
Energy loss (approximately 30 dB) occurs as sound waves transition from air to the lymphatic fluid of the inner ear due to differences in acoustic impedance, but this loss is compensated by the pressure-amplifying effects of the middle ear. Under typical sound intensities, the ossicular chain moves as a cohesive system. At sound intensities reaching 150 dB, the ossicular chain no longer moves as a unit due to the resistance of the stapes footplate and the damping effect at the incudostapedial joint. In such cases, vibration amplitude decreases progressively from the malleus to the incus and stapes. At low and moderate sound intensities, the stapes footplate vibrates along a vertical axis through its posterior crus, with larger anterior displacement compared to its posterior section. This vibration causes back-and-forth motion of the perilymph in the vestibular duct. When sound intensities approach the pain threshold, the stapes footplate rotates along its anterior-posterior axis. In this case, perilymph motion is localized near the oval window, and vibrations occur between the upper and lower edges of the stapes footplate. This mechanism prevents excessive displacement of the basilar membrane from intense sound stimulation, thereby reducing the risk of inner ear damage.
Physiological Functions of the Round Window
The round window is located at the beginning of the scala tympani and has an area of approximately 2 mm2. It is thin and possesses a certain degree of elasticity. The perilymph inside the bony labyrinth is almost incompressible, so when the stapes moves inward, vibrations are transmitted through the perilymph of the scala vestibuli, helicotrema, and scala tympani, ultimately causing the membrane of the round window to bulge outward. This mechanism permits the round window to serve as a pressure-relief structure, facilitating the transmission of sound waves within the perilymph. However, under pathological conditions (e.g., tympanic membrane perforation), the round window ceases to serve as a release valve for the bony cochlea and instead becomes a pathway for sound waves to enter the inner ear. Vibrations of the round window membrane in such cases induce disturbances in the perilymph of the scala tympani, which interfere with the perilymph vibrations in the scala vestibuli generated by stapes movement. This interference disrupts the propagation of vibrations along the basilar membrane, thereby leading to hearing loss.
Physiological Functions of the Tympanic Muscles
The contraction of tympanic muscles alters the sound transmission characteristics of the middle ear. The tympanic muscles include the tensor tympani muscle and the stapedius muscle. The tensor tympani is innervated by the trigeminal nerve. Its contraction pulls the handle of the malleus and the tympanic membrane inward, increasing the tension of the tympanic membrane and pushing the stapes footplate into the oval window, thereby raising the pressure in the perilymph of the inner ear. The stapedius muscle, innervated by the facial nerve, retracts the head of the stapes posteriorly during its contraction, causing the anterior portion of the stapes footplate to tilt outward and reducing perilymph pressure. These two muscles act in concert to protect the cochlea from damage.
The tensor tympani has a higher reflex threshold for sound stimuli compared to the stapedius muscle, meaning the stapedius muscle plays a primary role in reflexive contractions triggered by sound. Within the human auditory range, these middle ear muscles respond to most sound frequencies, but their reflex is most effective within the frequency range of 2,000–3,000 Hz. The intensity of sound required to trigger the reflex also varies by frequency, with the stapedius muscle reflex threshold generally ranging between 70–90 dB for sounds within the 250–4,000 Hz range.
The middle ear muscle reflex exhibits a latency period, which shortens as the stimulus intensity increases. When the tympanic muscles contract, the tension of the tympanic membrane increases, the connections between the ossicles tighten, and the stiffness of the ossicular chain rises. These changes reduce the efficiency of sound transmission in the middle ear, particularly for low-frequency sounds, with a sound attenuation of approximately 10–20 dB. The protective effect of the reflex is more pronounced for low-frequency sounds compared to high-frequency sounds. However, due to the latency period of the reflex, it offers limited protection against sudden explosive noises.
In clinical hearing assessments, the physiological properties of the stapedius muscle reflex triggered by sound stimuli are often used as a basis for diagnosis and differential diagnosis. However, non-auditory factors may also cause the contraction of middle ear muscles. Examples include:
- Spontaneous contraction;
- Body movement;
- Vocalization;
- Facial muscle movement (which only induces contraction of the tensor tympani);
- Stimulation of the external auditory canal;
- Voluntary contraction.
Physiological Functions of the Eustachian Tube
The Eustachian tube generally maintains a closed state but is capable of opening. Its physiological roles are as follows:
Balancing Pressure Between the Middle Ear and the External Environment
The pharyngeal opening of the Eustachian tube normally remains closed due to the elasticity of the tube walls, the pressure exerted by the surrounding tissues, and the traction from the pharyngeal region. During actions such as swallowing, yawning, or sneezing, the Eustachian tube opens to regulate air pressure within the tympanic cavity, ensuring it remains balanced with the external atmospheric pressure. This pressure equilibrium preserves the normal functioning of the middle ear's sound transmission apparatus, thereby facilitating sound wave conduction. Changes in air pressure can affect the opening and closing of the Eustachian tube. When the pressure within the tympanic cavity exceeds external atmospheric pressure, gas escapes more easily through the Eustachian tube. Conversely, when external atmospheric pressure exceeds the pressure within the tympanic cavity, gas entry into the middle ear is more difficult. The tensor veli palatini, levator veli palatini, and salpingopharyngeus muscles contribute to the opening of the Eustachian tube, with the tensor veli palatini playing the most significant role.
Drainage Function
The goblet cells and mucus glands located within the mucosa of the tympanic cavity and the Eustachian tube produce mucus, which is continuously cleared into the nasopharynx by the ciliary motion of the Eustachian tube's epithelial lining.
Sound Insulation and Noise Reduction
The closed state of the Eustachian tube prevents the direct transmission of sounds such as speech or breathing noises into the tympanic cavity, which could otherwise cause vibrations of the tympanic membrane. When the Eustachian tube remains abnormally open (e.g., due to patulous Eustachian tube dysfunction), sound waves can directly enter the tympanic cavity through the tube and vibrate the tympanic membrane, causing patients to perceive their own breathing sounds, which may be distressing.
The middle ear segment, located in the outer one-third of the Eustachian tube, is in an open state and has a gradually narrowing funnel-like shape. Its surface is lined with partially folded mucosa resembling a sound-absorbing structure. This arrangement absorbs sound waves generated within the tympanic cavity due to vibrations of the round window membrane and the tympanic membrane, providing a noise-reducing effect.
Prevention of Retrograde Infection
The cartilaginous segment of the Eustachian tube has relatively thick mucosa, with loose connective tissue in the submucosa that forms folds on the mucosal surface. These folds act as one-way valves. Combined with the ciliary motion of the epithelial lining, these features help prevent retrograde movement of liquid, foreign matter, or infection from the nasopharyngeal region into the tympanic cavity.
Physiology of the Cochlea
Auditory Sensing Function of the Cochlea
Sound wave vibrations cause the basilar membrane to oscillate, which propagates along the membrane in the form of a traveling wave. The propagation of sound waves on the basilar membrane follows the principles of the traveling wave theory. As the vibration moves from the base to the apex of the cochlea, the amplitude gradually increases, reaching its maximum at the location where the resonance frequency of the basilar membrane matches the frequency of the sound wave. Beyond this point, the amplitude rapidly decreases, and the displacement stops entirely a short distance further along. The time required for a traveling wave to traverse the human basilar membrane is approximately 3 milliseconds.
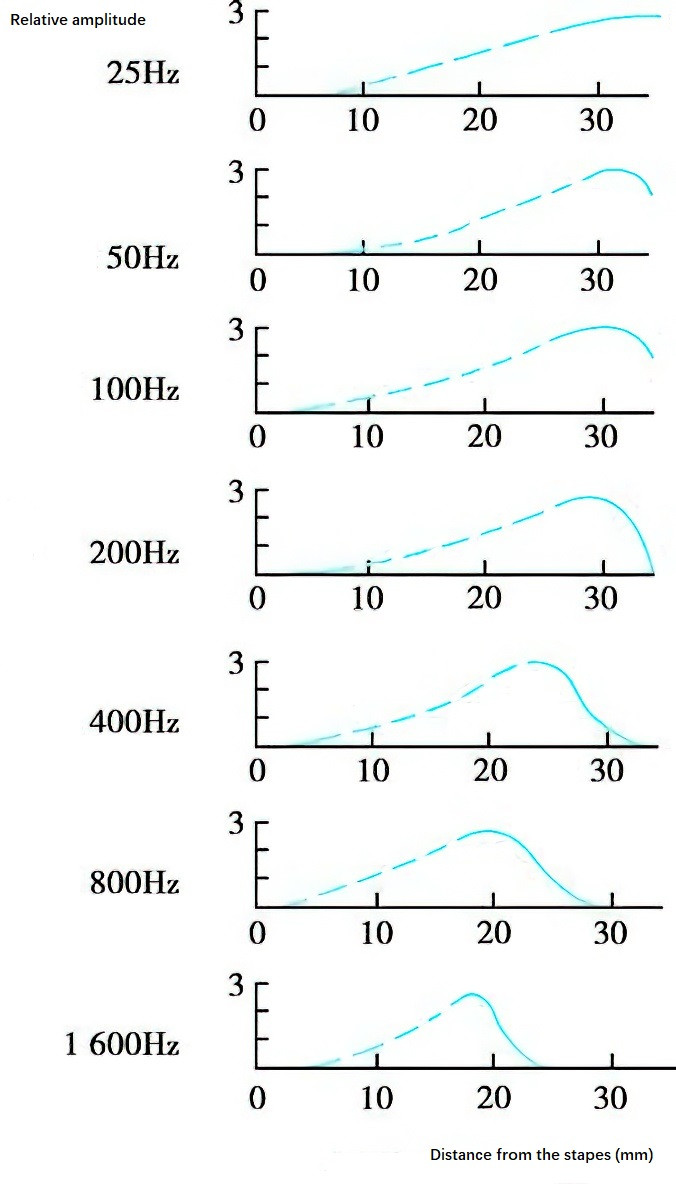
Figure 7 Graph of basilar membrane displacement induced by sound waves of different frequencies
The location of maximum vibration amplitude on the basilar membrane is frequency-dependent, meaning that every sound frequency corresponds to a specific location on the membrane: high-frequency sounds produce maximum vibration near the cochlear base close to the oval window, low-frequency sounds produce maximum vibration near the cochlear apex, and mid-frequency sounds resonate in the middle segment of the basilar membrane.
This demonstrates that high-frequency sound waves cause vibrations only in the basilar membrane near the oval window, whereas low-frequency sound waves travel from the cochlear base to the apex and result in the displacement of a larger portion of the basilar membrane, with the maximum amplitude at the resonance point. The basilar membrane near the base responds to sound waves of all frequencies, while the membrane near the apex responds only to low-frequency sound waves. Specific regions of the basilar membrane are tuned to different frequencies: the cochlear base is sensitive to high frequencies, the cochlear apex to low frequencies, frequencies below 800 Hz are detected at the apex, and 2,000 Hz is detected midway between the helicotrema and the stapes footplate. This behavior reflects the passive mechanical properties of the basilar membrane and the classic traveling wave mechanism.
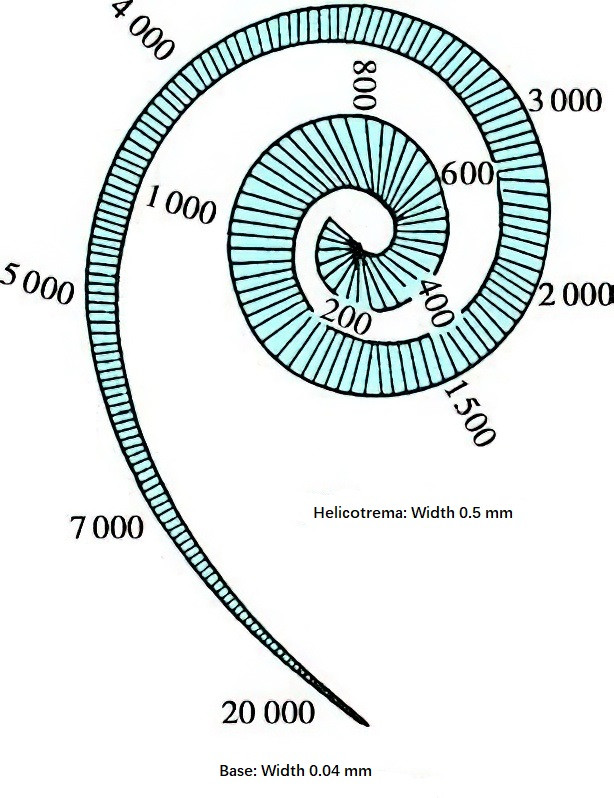
Figure 8 Frequency distribution of the basilar membrane (Unit: Hz)
When sound waves enter the cochlear perilymph and traveling waves induce upward or downward displacement of the basilar membrane, the membrane, which is attached to the bony spiral lamina along its inner edge, moves vertically along a different axis from the tectorial membrane. This movement results in a sliding action, or shearing motion, between the tectorial membrane and the reticular lamina, thereby generating a shearing force. This shearing force bends or deflects the stereocilia of hair cells. In this process, K+ channels at the tips of the hair cells open, allowing K+ from the endolymph to flow into the cells, causing depolarization. Depolarization subsequently opens Ca2+ channels in the cell membrane, promoting the influx of Ca2+ into the hair cell, which triggers the release of neurotransmitters. This release induces nerve impulses in the cochlear nerve endings attached to the base of the hair cells, which are transmitted through central auditory pathways to the auditory cortex, resulting in the perception of sound.
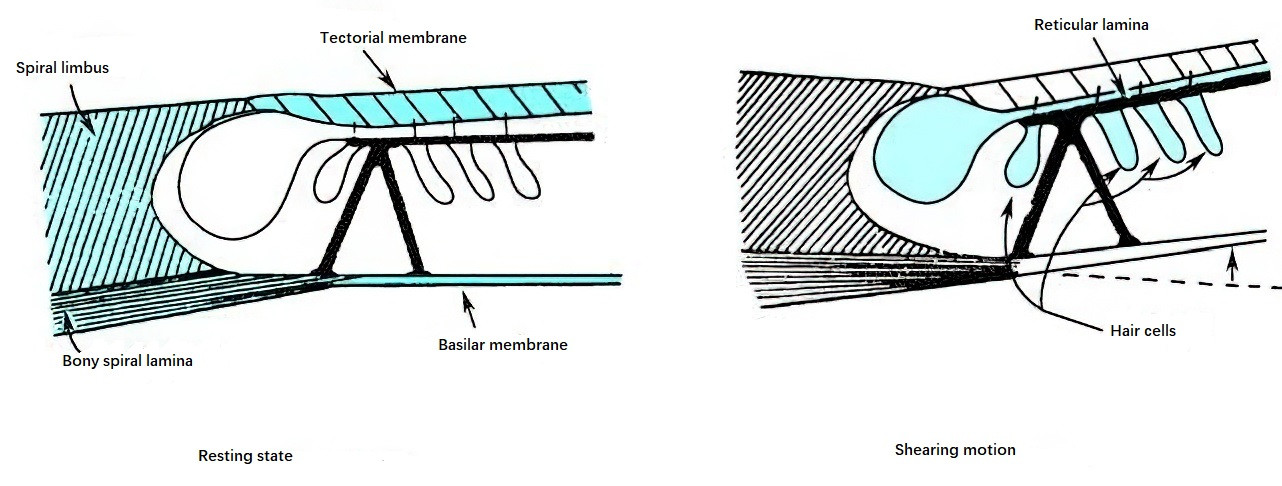
Figure 9 Shearing motion between the reticular lamina and the tectorial membrane causing deflection of hair cell stereocilia
Frequency Encoding Function of the Cochlea
The passive mechanical properties of the basilar membrane, determined by its mass and stiffness gradients, establish a correspondence between the frequency of the sound stimulus and the responsive regions of the cochlear basilar membrane. Research has demonstrated that the cochlea possesses a refined frequency analysis capability, with frequency tuning curves far superior to the results observed by Békésy in cadaver studies. This suggests that the passive mechanical characteristics of the basilar membrane and the classic traveling wave mechanism are not the sole contributors to cochlear frequency analysis or tuning. Active mechanisms within the cochlear spiral organ, potentially linked to energy metabolism, may also contribute to frequency encoding.
Otoacoustic Emissions (OAE)
Researchers have recorded otoacoustic emissions (OAE) from human ears, confirming the existence of active energy release within the cochlea. The process of otoacoustic emission involves the conversion of bioelectrical energy back into mechanical (sound-frequency) energy, proving that the cochlea functions as a bidirectional transducer. Otoacoustic emissions are sound-frequency energy recorded in the external auditory canal, originating from the cochlear spiral organ in individuals with normal hearing. They are generally thought to arise from the active motility of outer hair cells.
Studies have demonstrated that the outer hair cell walls contain contractile proteins such as actin and myosin, which serve as the structural foundation for the active motility of these cells. Recent discoveries have revealed that individual outer hair cells in the cochlea exhibit active elongation and contraction, occurring in both slow and fast modes. Slow outer hair cell motility may regulate the mechanical properties of the basilar membrane, while fast motility enhances the gain of incoming sound signals. This increases cochlear sensitivity and sharpens frequency selectivity (or tuning). Both the oscillation of the basilar membrane and the nonlinear response of hair cell membrane potentials to sound stimuli provide evidence for the active mechanisms of the cochlea.
The physiological significance of this active cochlear mechanism lies in its ability to enhance the basilar membrane's mechanical response to sound stimulation, thereby improving frequency resolution and auditory sensitivity. Temporary threshold shifts following intense auditory stimulation and the recruitment phenomena observed in cochlear hearing loss are related to dysfunctions in these active cochlear mechanisms.
Regulation of Cochlear Function by Efferent Nerves
In addition to afferent nerve fibers, the cochlear spiral organ is also connected to efferent nerve fibers and is regulated by the efferent auditory system. These efferent nerve fibers innervating the spiral organ originate from neurons near the superior olivary nucleus and are referred to as the olivocochlear bundle, which mainly targets outer hair cells. It is currently believed that the olivocochlear bundle functions to suppress afferent neural potentials generated by low- and medium-intensity sound stimuli, thereby enhancing the auditory system's ability to discern higher-intensity sound information.
Bioelectric Phenomena in the Cochlea
Resting Potentials of Cells and Endocochlear Potential
The resting potential of a cell refers to the electrical potential difference between the inside and outside of cells in the spiral organ, which is caused by differences in the concentrations of ions on either side of the cell membrane. This results in the interior of the membrane having a negative potential, while the exterior has a positive potential.
The endocochlear potential (EP), also known as the endolymphatic potential, refers to the potential difference between the endolymph in the cochlear duct and the perilymph in the scala tympani. It has been demonstrated that this potential originates from the stria vascularis cells on the lateral wall of the cochlear duct. EP plays a role in facilitating the transformation of sound energy into neural impulses in auditory sensory receptors. EP decreases rapidly under conditions of hypoxia or when metabolic inhibitors are applied.
Cochlear Microphonic Potential (CM)
The cochlear microphonic potential is an alternating current potential generated by the cochlea in response to sound stimuli. It originates at the boundary between the cuticular plate on top of hair cells and the endolymph. According to the variable resistance theory, the cuticular plate of hair cells has a significant resistance. When sound causes the basilar membrane to vibrate, this resistance changes as the stereocilia bend. When the stereocilia bend in one direction, the resistance increases, whereas bending in the opposite direction decreases the resistance. The current flowing through the cell changes accordingly. As stereocilia oscillate alternately, an alternating current voltage is produced across the two sides of the hair cell cuticular plate, generating the CM. It is now believed that the CM is primarily produced by the outer hair cells.
Summating Potential (SP)
The summating potential is a direct current potential generated by hair cells in response to sound stimulation, and it originates from the inner hair cells.
Cochlear Nerve Action Potential (AP)
The cochlear nerve action potential is produced by cochlear nerves in response to sound stimulation and serves to transmit auditory information.
Auditory Central Physiology
Many mechanisms underlying auditory central physiology remain unclear. Compared to the peripheral auditory system, the central auditory system is significantly more complex in terms of structure, function, activity patterns, mechanisms, and regulatory processes. The central auditory structures include the cochlear nucleus, superior olivary nucleus, lateral lemniscus nucleus, inferior colliculus, medial geniculate body, auditory radiation, and auditory cortex. The subcortical levels and pathways of the auditory system are more numerous than those of other sensory systems. While other sensory systems typically have only one or two subcortical relay stations between the input nerves and the thalamus, the auditory system has four subcortical relay nuclei (cochlear nucleus, superior olivary nucleus, lateral lemniscus nucleus, and inferior colliculus).
The complexity of the central auditory structure is further reflected in the subdivision of each relay nucleus into smaller regions based on anatomical location. Each subdivision can be further divided into smaller zones according to the types and connections of neurons within them.
A single neuron in the cochlear nucleus can receive inputs from multiple afferent fibers, while each afferent fiber from the auditory nerve can branch to innervate multiple neurons in the cochlear nucleus. Branches from the same fiber can even converge on the same neuron, forming synaptic connections in different ways. This anatomical arrangement reflects both convergent and divergent modes of connection, which show that afferent information undergoes corresponding transformations after entering the central system from the periphery. The processes of convergence and divergence do not merely rearrange information; instead, they result in qualitative transformations. Through analysis and integration, incoming information is synthesized into higher-order patterns, encoded, and transmitted upward. This type of processing occurs at progressively higher levels as the central auditory pathway ascends.
Through repeated processing at different levels of the auditory pathway, auditory information is progressively transformed from simple parameters like frequency and intensity into more complex attributes, such as sound features and sound images. Ultimately, these attributes are converted into forms that facilitate perception, recognition, understanding, and cognitive processing.
The auditory central pathway also interacts with the facial nerve, trigeminal nerve, abducens nerve, and other cranial nerves, as well as autonomic nerve nuclei and anterior horn cells of the spinal cord. These connections allow strong auditory stimuli to elicit reflexive responses, such as blinking, outward eye movements, head turning toward the sound source, contractions of middle ear muscles, constriction of finger blood vessels, and changes in skin electrical potential.