Acute respiratory distress syndrome (ARDS) refers to acute hypoxemic respiratory failure caused by inflammatory damage to the diffuse alveolar-capillary endothelium and alveolar epithelial cells due to various etiologies such as severe infections, shock, trauma, and burns.
Etiology and Risk Factors
The causes and risk factors for ARDS are diverse and can be categorized into direct and indirect pulmonary injuries. Approximately 50% of ARDS cases are linked to pulmonary factors, with sepsis being the most common cause and risk factor. About 40% of patients with sepsis in ICUs develop ARDS. The risk of ARDS increases with the accumulation of risk factors. Surgical procedures are also common high-risk factors, and the incidence of ARDS can reach 10% to 20% following valve replacement surgery, abdominal aortic aneurysm surgery, or major abdominal surgeries complicated by intra-abdominal hypertension.
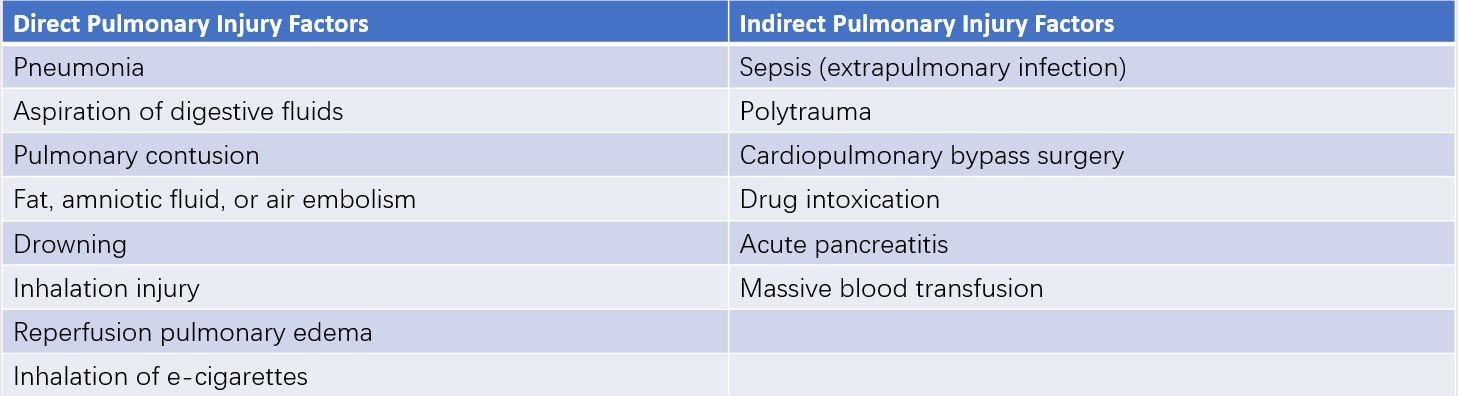
Table 1 Causes and risk factors of ARDS
Pathology
The complex pathological changes in ARDS remain incompletely understood. The typical pathological changes in ARDS are divided into three phases: the exudative phase, the proliferative phase, and the fibrotic phase. These stages are interrelated, partially overlap throughout the disease course, and exhibit heterogeneity.
Exudative Phase
The primary manifestation is damage to the diffuse alveolar-capillary barrier. Type I alveolar epithelial cells detach, intercellular gaps widen, the basement membrane disintegrates, and fluid from inside the vasculature leaks out, forming hyaline membranes. Focal or widespread alveolar collapse is characteristic of this phase.
Proliferative Phase
This phase is characterized by massive proliferation of Type II alveolar epithelial cells, alveolar sac and duct fibrosis, intimal proliferation of muscular arterioles, and narrowed lumens.
Fibrotic Phase
In late-stage ARDS patients, diffuse and irregular pulmonary fibrosis typically occurs. Between 15% and 40% of ARDS patients in the fibrotic phase succumb to refractory respiratory failure.
Pathophysiology
Reduction in Lung Volume
The lungs in ARDS are often referred to as "small lungs" or "baby lungs." In severe ARDS cases, the alveoli engaged in ventilation may only account for one-third of the normal alveoli.
Decreased Lung Compliance
A significant feature of ARDS is reduced lung compliance. This reduction is primarily related to increased surface tension caused by decreased surfactant, as well as atelectasis and pulmonary edema, which reduce lung volume.
Ventilation/Perfusion (V/Q) Mismatch
V/Q mismatch is the primary cause of hypoxemia in ARDS. Due to the heterogeneity of lung changes in ARDS, both increased and decreased V/Q ratios can coexist in different affected regions.
Pulmonary Hypertension
In early-stage ARDS, factors such as hypoxia, vasoconstrictive mediators (e.g., thromboxane A2 (TXA2), tumor necrosis factor-alpha (TNF-α)), and reduced nitric oxide production lead to pulmonary artery spasm and elevated pulmonary artery pressure. In late-stage ARDS, irreversible pulmonary hypertension arises due to structural changes such as smooth muscle proliferation in pulmonary arterioles and the transformation of non-muscular arterioles into muscular arterioles.
Clinical Features and Investigations
Symptoms and Signs
ARDS typically appears within one week of the onset of the primary condition or trigger. Clinical manifestations include progressive dyspnea and hypoxemia, along with tachypnea, tachycardia, sweating, and restlessness. As hypoxemia worsens in severity and duration, extrapulmonary organ damage may occur, potentially leading to multiple organ dysfunction or failure. In addition to the above symptoms and signs, manifestations of the underlying primary disease are also present.
Imaging
X-ray, CT, and ultrasound are common imaging modalities used for ARDS evaluation. Imaging findings in ARDS are non-specific and may include ground-glass opacity, interstitial edema with pulmonary infiltration, consolidation, atelectasis, and pleural effusion. These findings primarily appear in areas associated with disease, resulting from pulmonary edema, inflammatory interstitial edema, and pulmonary capillary congestion. Although ARDS imaging lacks specificity, it remains an important diagnostic tool and plays a significant role in differential diagnosis and guiding clinical management.
Arterial Blood Gas Analysis
Arterial blood gas analysis is a key test for evaluating pulmonary gas exchange. The ratio of arterial oxygen partial pressure (PaO2) to the fraction of inspired oxygen (FiO2) is termed the oxygenation index, which serves as an indicator of hypoxemia severity in ARDS. In early-stage ARDS, respiratory alkalosis may occur due to increased respiratory rate. In later stages, as ineffective ventilation worsens, elevated carbon dioxide levels may appear.
Bronchoscopy
Bronchoscopy can be used to obtain lavage fluid or sputum samples for cytological, microbiological, biochemical, and proteomic analyses through techniques such as bronchoalveolar lavage or protective bronchial brushing. This is an important method for identifying intrapulmonary causes of ARDS.
Hemodynamic Monitoring
Hemodynamic monitoring is a crucial component in ARDS diagnosis and treatment, particularly for distinguishing cardiogenic pulmonary edema from ARDS and guiding fluid management in patients with ARDS.
Diagnosis
ARDS is suspected in cases involving systemic infections, shock, severe pulmonary infections, massive transfusion, acute pancreatitis, or severe trauma leading to progressive dyspnea and hypoxemia that cannot be corrected by conventional oxygen therapy. Such symptoms typically occur within one week, accompanied by imaging findings of bilateral patchy infiltrates on the lungs, which cannot be explained by heart failure-induced pulmonary edema. Diagnosis of ARDS requires fulfillment of all the following criteria:
- Respiratory symptoms must begin within one week of a known clinical insult or the onset of new or worsening symptoms has occurred in the past week.
- Chest X-ray or CT scan should show bilateral lung opacities consistent with pulmonary edema that cannot be fully attributed to pleural effusion, lung collapse, or pulmonary nodules.
- The patient's respiratory failure must not be fully explained by cardiac failure or fluid overload.
- Moderate to severe oxygenation impairment is present, defined using the PaO2/FiO2 ratio (partial pressure of arterial oxygen to fraction of inspired oxygen):
- Mild ARDS: PaO2/FiO2 ratio >200 mmHg and ≤300 mmHg while ventilated with a positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) of ≥5 cmH2O.
- Moderate ARDS: PaO2/FiO2 ratio >100 mmHg and ≤200 mmHg with PEEP ≥5 cmH2O.
- Severe ARDS: PaO2/FiO2 ratio ≤100 mmHg with PEEP ≥5 cmH2O.
Differential diagnoses include other conditions causing hypoxemia, such as cardiogenic pulmonary edema, nephrotic syndrome, vasculitis, non-cardiogenic pulmonary edema due to systemic diseases like cirrhosis, as well as acute pulmonary embolism, acute exacerbation of chronic obstructive pulmonary disease, and idiopathic pulmonary interstitial fibrosis.
Treatment
The general principles of ARDS treatment involve correcting hypoxemia, improving systemic oxygen delivery, maintaining tissue perfusion, preventing further organ damage, and avoiding iatrogenic injuries such as ventilator-induced lung injury (VILI).
Etiological Treatment
The underlying cause is a key factor influencing the progression and outcome of ARDS. Prompt and effective management of the primary cause is critical in ARDS treatment. This includes drainage and control of infection foci, timely and rational use of antibiotics, and treatment of conditions like intra-abdominal hypertension and acute pancreatitis. However, controlling some complex etiologies may prove challenging within a short period, making the treatment of underlying causes difficult in ARDS cases.
Oxygen Therapy
Oxygen therapy is an essential intervention to correct hypoxemia in ARDS patients. The methods of oxygen delivery include nasal cannula/mask oxygenation, high-flow nasal cannula (HFNC), non-invasive mechanical ventilation, and invasive mechanical ventilation. Due to the pathophysiological changes in ARDS, low-flow oxygen delivery through nasal cannula or mask is often insufficient to address hypoxemia. Mechanical ventilation becomes the most commonly used method of oxygen therapy. It effectively reduces respiratory workload, corrects hypoxemia, improves systemic oxygenation, and protects extrapulmonary organs by maintaining effective inspiratory and expiratory positive airway pressure, thereby alleviating atelectasis and reducing intrapulmonary shunting.
Prone Ventilation
Prone ventilation reduces the pressure exerted by the gravitational effect of the body on alveoli in the gravity-dependent dorsal regions of the lungs, allowing a more uniform pleural pressure distribution between the ventral and dorsal areas. It also reduces the compression of parts of the lungs caused by the heart and mediastinum, aiding the recruitment of collapsed alveoli in the dorsal regions while decreasing alveolar dead space in the ventral areas of the lungs. This leads to a more even distribution of ventilation. In cases of moderate to severe ARDS with refractory hypoxemia, prone ventilation significantly improves oxygenation and outcomes.
Extracorporeal Membrane Oxygenation (ECMO)
Extracorporeal membrane oxygenation (ECMO) is a form of extracorporeal lung assist (ECLA) technology. Venovenous ECMO (VV-ECMO) is primarily employed to partially or fully replace lung function, allowing the lungs to rest adequately and providing time for lung repair and treatment of the underlying disease. Early application of ECMO can be implemented for patients with severe ARDS who cannot meet adequate oxygenation and ventilation needs through conventional respiratory support methods.
Fluid Management
Patients with ARDS often experience shock due to factors such as increased intrathoracic pressure caused by mechanical ventilation, reduced venous return, primary infectious diseases, or sepsis. However, in the context of abnormal alveolar-capillary barrier permeability in ARDS, even a modest increase in pulmonary hydrostatic pressure can worsen pulmonary edema. Excess fluid accumulation is detrimental to the outcomes of ARDS patients. Dynamic hemodynamic monitoring should guide a restrictive fluid therapy strategy, avoiding unnecessary fluid resuscitation and positive fluid balance.
Sedation, Analgesia, and Neuromuscular Blocking Agents
Sedatives and analgesics are routinely used in ARDS patients undergoing endotracheal intubation or ECMO to reduce oxygen consumption, improve oxygenation, enhance comfort, and improve synchronization between the patient and ventilator. These measures also mitigate lung injury caused by excessive spontaneous breathing efforts. In cases of moderate to severe ARDS where deep sedation and analgesia cannot sufficiently suppress excessive spontaneous breathing, short-term administration of neuromuscular blocking agents may be considered.
Additional Supportive Measures and Prevention of Complications
For ARDS patients experiencing excessive inflammatory responses, difficulties in fluid management, or severe metabolic imbalances, blood purification therapy may be considered. ARDS patients often manifest a systemic stress response, entering a hypermetabolic state characterized by increased endogenous nitrogen loss and electrolyte imbalances. Mechanical ventilation or blood purification techniques can further accelerate energy consumption. Early initiation of nutritional support is advisable whenever no absolute contraindications exist. Appropriate nutritional routes should be based on gastrointestinal function. Energy supply should be adequate and complemented with micronutrients and vitamins, along with carbohydrates, fats, and amino acids.
Additionally, ARDS patients are prone to complications such as deep vein thrombosis (DVT) in the lower limbs due to factors like prolonged bed rest and a hypercoagulable state. Preventive measures, including pharmacological or mechanical prophylaxis, should be tailored to the individual patient's risk factors and bleeding risk.