Acute renal failure (ARF) refers to a rapid decline in kidney function occurring over a short period (ranging from a few hours to days). It is characterized by a reduction in solute clearance and the glomerular filtration rate (GFR), leading to a clinical syndrome marked by water, electrolyte, and acid-base imbalances, as well as the accumulation of nitrogenous waste products. In recent years, ARF has been categorized under the term acute kidney injury (AKI), which places greater emphasis on the importance of early diagnosis, prevention, and management of this syndrome. The current definition and staging criteria for AKI are widely utilized in both clinical practice and research.

Table 1 Definition of AKI
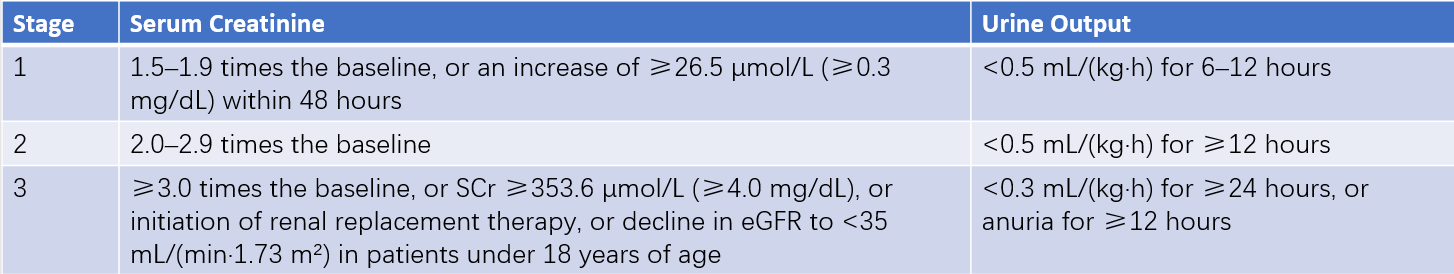
Table 2 Staging criteria for AKI
The incidence and mortality of AKI remain consistently high. Approximately 10–15% of hospitalized patients experience AKI, and the incidence is even greater in intensive care units (ICUs), where more than 50% of critically ill patients develop AKI. Of these cases, around 25–75% are associated with sepsis or septic shock, with mortality rates ranging from 11% to 77%.
Etiology and Classification
The causes of AKI or ARF can be broadly categorized into three types: prerenal, renal (intrinsic), and postrenal. In a narrower sense, AKI often refers to acute tubular necrosis (ATN).
Prerenal Causes
The underlying causes include acute reductions in blood volume due to various factors; decreased cardiac output caused by conditions such as congestive heart failure, acute myocardial infarction, severe arrhythmias, cardiac tamponade, or pulmonary embolism; reductions in effective circulating blood volume or its redistribution due to systemic diseases such as septic shock, anaphylaxis, or hepatorenal syndrome; and increased renal vascular resistance caused by vascular disease or medications. These factors lead to a state of renal hypoperfusion, where the GFR cannot be maintained, resulting in oliguria. At this stage, renal parenchyma remains unscathed, representing a functional alteration. However, if this condition is not addressed promptly, progressive reductions in renal blood flow may lead to acute tubular necrosis and the onset of ARF.
Renal (Intrinsic) Causes
Intrinsic causes are primarily attributed to ischemia and nephrotoxin-induced injury, leading to acute damage to the renal parenchyma, with acute tubular necrosis being the most common type. The damage may involve the glomeruli, renal tubules, interstitium, or renal vasculature. Numerous factors can lead to kidney ischemia, such as massive hemorrhage, septic shock, or severe anaphylactic reactions. Nephrotoxic substances include aminoglycosides (such as gentamicin and kanamycin), heavy metals (including bismuth, mercury, aluminum, and arsenic), other drugs (such as radiocontrast agents, acyclovir, cisplatin, cyclosporine, and amphotericin B), organic solvents (such as carbon tetrachloride, ethylene glycol, benzene, and phenol), and biological toxins (such as snake venom and mushroom toxins). The effects of ischemia and nephrotoxins on the kidneys often overlap, as observed in conditions such as crush syndrome and septic shock.
Postrenal Causes
Postrenal causes arise from urinary tract obstruction, including conditions affecting both kidneys or ureters, as well as pelvic tumors compressing the ureters, which result in hydronephrosis above the obstruction. Other causes include bladder stones, tumors, prostatic hyperplasia, prostate cancer, and urethral strictures leading to bilateral lower urinary tract obstruction and a sudden decline in renal function.
Clinical Manifestations
Acute renal failure is classified into oliguric and non-oliguric types. Oliguric ARF is further divided into the oliguric (or anuric) phase, diuretic phase, and recovery phase during its clinical course.
Oliguric (or Anuric) Phase
This phase represents the primary stage of the disease course and typically lasts 7–14 days (averaging 5–6 days but potentially extending beyond one month in severe cases). A longer oliguric phase is associated with more severe disease and a poorer prognosis.
Reduced Urine Output
Patients exhibit a sudden or gradual reduction in urine output, with 24-hour urine volumes below 400 mL considered oliguria and less than 100 mL considered anuria. Non-oliguric acute renal failure refers to cases in which urine output remains above 400 mL per day (possibly even 1,000–2,000 mL) despite progressing nitrogenous waste accumulation. The mechanisms underlying non-oliguric ARF remain unclear, with three main explanations proposed:
- Damage to individual nephron units may vary, with some nephrons maintaining blood flow and GFR yet showing significant tubular reabsorption dysfunction.
- All nephron units may experience similar levels of damage; however, tubular reabsorption impairment may be disproportionately severe compared to reductions in GFR.
- Impaired ability to create a hyperosmotic medullary environment may reduce water reabsorption in the loop of Henle.
In general, compared to oliguric ARF, non-oliguric ARF presents with milder clinical manifestations, slower progression, fewer complications such as severe fluid, electrolyte, and acid-base disturbances or gastrointestinal bleeding, and a lower incidence of high potassium levels. Nonetheless, non-oliguric ARF still carries a high mortality rate, reaching up to 26%, and requires clinical attention.
Progressive Azotemia
Progressive azotemia occurs as a result of decreased glomerular filtration rate (GFR), leading to an inability to excrete protein metabolic byproducts through the kidneys. This results in the accumulation of nitrogen-containing substances in the blood, a condition known as azotemia. When accompanied by fever, infection, or injury, there may be an increase in protein catabolism, causing blood urea nitrogen (BUN) and creatinine levels to rise more rapidly. In the context of azotemia, other toxic substances such as phenols and guanidines also accumulate in the blood, eventually leading to uremia. Clinical manifestations of uremia include nausea, vomiting, headache, irritability, fatigue, lethargy, confusion, and, in severe cases, coma.
Disorders of Water, Electrolyte, and Acid-Base Balance
Fluid Overload
Prolongation of the oliguric phase leads to significant water retention in the body, compounded by endogenous water production. This predisposes patients to fluid overload and, in severe cases, water intoxication. Serious complications may include hypertension, heart failure, pulmonary edema, and cerebral edema. Water intoxication is one of the leading causes of death in acute renal failure (ARF).
Hyperkalemia
In healthy individuals, 90% of potassium excretion occurs via the kidneys. During oliguria or anuria, potassium excretion is impaired. Conditions such as increased tissue catabolism (e.g., severe crush injury) facilitate the release of potassium from intracellular to extracellular compartments. Additionally, acidosis causes intracellular potassium to shift into the extracellular space. These processes can result in a rapid and dangerous rise in blood potassium levels, sometimes within hours, which is one of the common causes of death in ARF.
Hypermagnesemia
Under normal conditions, magnesium is approximately 60% excreted via feces and 40% via urine. In ARF, serum magnesium levels often parallel potassium levels. Hypermagnesemia can produce electrocardiographic changes, including prolonged PR intervals, widened QRS complexes, and peaked T waves. Elevated magnesium levels may also cause neuromuscular transmission dysfunction, leading to symptoms such as hypotension, respiratory depression, numbness, muscle weakness, coma, or even cardiac arrest.
Hyponatremia and Hypochloremia
Hyponatremia and hypochloremia often occur simultaneously. Hyponatremia may result from dilution due to fluid overload (dilutional hyponatremia) or sodium loss caused by skin, gastrointestinal losses, or diuretic use. Severe hyponatremia can lead to decreased serum osmolality, causing water to shift into cells and resulting in cellular edema. Clinical manifestations include fatigue, drowsiness, disorientation, and, in severe cases, hypotonic coma. Hypochloremia is associated with vomiting, diarrhea, or the use of large doses of loop diuretics and is characterized by symptoms of metabolic alkalosis, including abdominal distension, shallow breathing, and convulsions.
Hyperphosphatemia and Hypocalcemia
Hyperphosphatemia occurs in ARF, with 60%–80% of phosphorus being excreted through the gastrointestinal tract and precipitating as insoluble calcium phosphate. This reduces calcium absorption, resulting in hypocalcemia. Low blood calcium levels can cause muscle twitching and intensify the cardiotoxic effects of hyperkalemia.
Metabolic Acidosis
Metabolic acidosis is a key pathophysiological feature of the oliguric phase of ARF. Hypoxia leads to increased anaerobic metabolism, and the excretion of non-volatile acidic metabolites such as inorganic phosphates becomes impaired. Additional factors contributing to acidosis include damage to renal tubules, loss of bicarbonate and sodium ions, and reduced secretion of hydrogen ions as well as their combination with ammonia (NH3). The accumulation of acidic metabolites in the body results in a reduction in blood bicarbonate (HCO3⁻) concentration, thereby exacerbating metabolic acidosis and worsening hyperkalemia. Clinical manifestations of metabolic acidosis include deep, rapid breathing, a fruity odor on the breath due to ketone exhalation, facial flushing, chest tightness, dyspnea, drowsiness, and altered levels of consciousness. In severe cases, hypotension, arrhythmias, and cardiac arrest may occur.
Systemic Complications
Cardiovascular System
Complications may include hypertension, acute pulmonary edema, heart failure, arrhythmias, and pericarditis.
Gastrointestinal System
Common symptoms include loss of appetite, nausea, vomiting, abdominal distension, and diarrhea. Gastrointestinal bleeding and jaundice may also occur.
Nervous System
Fatigue, mental dullness, and, in severe cases, changes in consciousness such as lethargy, agitation, or even coma, are indicative of disease severity.
Hematologic System
Anemia and disseminated intravascular coagulation (DIC) are common. The degree of anemia is closely related to the underlying cause, the duration of the disease, and the presence of bleeding complications.
Polyuric Phase
The polyuric phase begins when the urine output increases to more than 800 mL within 24 hours, typically occurring 7 to 14 days after the onset of oliguria or anuria. This phase usually lasts approximately 14 days, during which daily urine output can exceed 3,000 mL. During the first week of this phase, renal tubular epithelial cell function remains incompletely restored. Although urine output increases significantly, levels of blood urea nitrogen, creatinine, and potassium continue to rise, and symptoms of uremia persist. This stage is referred to as the early polyuric phase. As renal function further recovers and urine output increases substantially, imbalances such as hypokalemia, hyponatremia, hypocalcemia, hypomagnesemia, and dehydration may develop. At this stage, patients remain in a state of azotemia and water-electrolyte imbalance. Improvement is indicated by a decline in blood urea nitrogen and creatinine levels, marking the transition to the late polyuric phase.
Recovery Phase
In the early stages of recovery, patients may exhibit no symptoms or may experience general weakness, fatigue, and weight loss. Glomerular filtration function commonly returns to normal within 3 to 6 months; however, some patients may exhibit impaired tubular concentration ability for over a year. Persistent non-recovery of kidney function suggests the presence of permanent renal damage, and in rare cases, fibrosis of renal tissue may lead to chronic renal insufficiency.
Diagnosis and Differential Diagnosis
The diagnosis and differential diagnosis of acute kidney injury (AKI) can be made based on the underlying disease, clinical presentation, laboratory findings, and imaging studies.
History and Physical Examination
A thorough history should include factors related to AKI, summarized into the following three categories: (1) the presence of prerenal factors, (2) potential causes of acute tubular necrosis, and (3) the presence of postrenal factors. It is also important to assess previous kidney diseases, renal vascular abnormalities, or the occurrence of AKI superimposed on preexisting chronic kidney disease (CKD). Assessment of generalized and limb edema and the level of jugular venous distension can provide insights into the etiology of AKI as well as the current status of fluid-electrolyte balance and cardiac function. Auscultation of the heart and lungs may help detect heart failure, pulmonary edema, or arrhythmias.
Urinalysis
Changes in urine color should be noted, as "cola-colored" urine may indicate hemolysis or severe soft tissue injury. In prerenal AKI, urine is concentrated, with high specific gravity and osmolality, whereas in renal AKI, urine is isotonic, with specific gravity ranging from 1.010 to 1.014. Urinalysis findings can include broad brown granular casts suggestive of acute tubular necrosis (ATN), large numbers of red blood cell casts and protein possibly indicative of acute glomerulonephritis, and the presence of white blood cell casts, suggestive of acute pyelonephritis. Distinct differences in urine characteristics are often observed between oliguria in prerenal AKI and the oliguria phase of ATN.
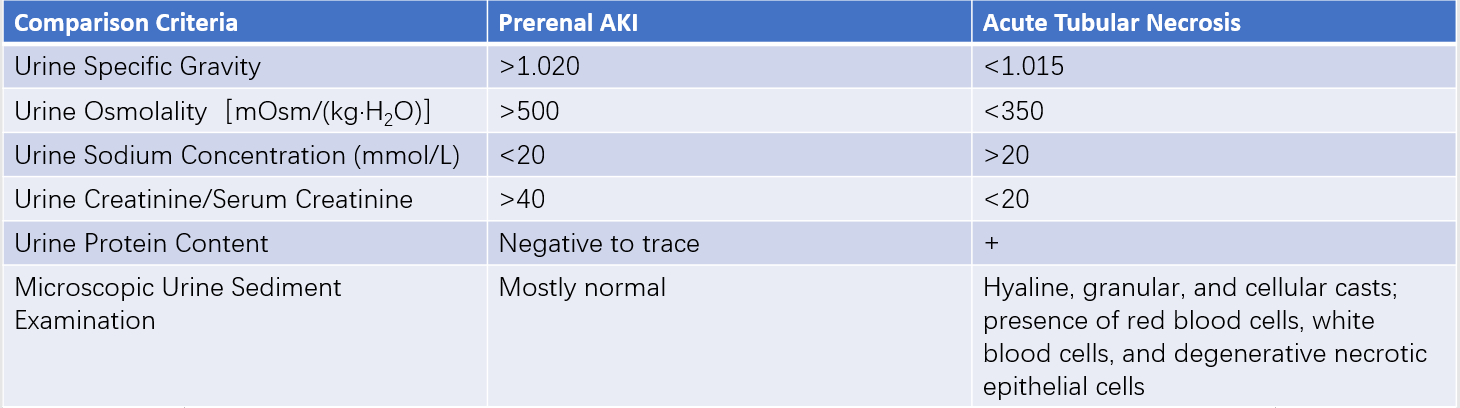
Table 3 Comparison of urinary changes in prerenal AKI and acute tubular necrosis during the oliguric phase
Blood Tests
A complete blood count may reveal significant eosinophilia, which suggests acute interstitial nephritis, while mild to moderate anemia may indicate fluid retention.
Acid-base balance and electrolyte levels should be monitored dynamically.
Blood urea nitrogen, creatinine, and creatinine clearance levels should be evaluated over time to monitor trends.
Imaging Studies
Imaging studies are particularly important for diagnosing postrenal AKI. Ultrasound (B-mode) studies can reveal kidney size and the presence of hydronephrosis. Plain abdominal X-rays or noncontrast CT scans can identify urinary calculi. In cases of suspected urinary obstruction, retrograde urography can be utilized. Magnetic resonance urography (MRU) can visualize the site and degree of urinary tract obstruction. X-rays or radionuclide studies can detect renal vascular obstruction, and renal angiography may confirm the diagnosis but requires careful consideration due to the nephrotoxicity of contrast agents.
Kidney Biopsy
Kidney biopsy is typically reserved for cases of intrinsic acute kidney failure with unclear etiology, such as glomerulonephritis, vasculitis, or allergic interstitial nephritis.
Treatment
The principles of treating acute kidney injury (AKI) include: (1) enhancing fluid management to maintain fluid balance; (2) maintaining internal homeostasis by regulating electrolyte and acid-base balance; (3) controlling infections; (4) utilizing renal replacement therapy to remove toxins and facilitate the repair of damaged cells; (5) identifying risk factors for AKI early and actively addressing the primary disease.
Treatment During the Oliguric Phase
Fluid Management
Rational fluid management is critical during both the oliguric and polyuric phases, whether for preventing the progression of AKI or promoting recovery. In mild AKI, the focus is on replenishing volume, improving hypoperfusion, and preventing further episodes of hypoperfusion. In more severe cases of AKI or acute renal failure (ARF), where diuretic resistance is common, strict limits on water and sodium intake are needed during the oliguric phase. After correcting the pre-existing fluid deficit, a "input equals output" approach is followed. The daily fluid infusion volume equals the previous day's urine output plus insensible fluid losses (approximately 400 mL). Insensible losses are estimated by subtracting endogenous water production (300 mL) from water lost through skin and respiration (700 mL). Observable losses such as those from stool, vomitus, exudates, and drainage are also included. For febrile patients, an additional 100 mL of fluid is added for every 1°C increase in body temperature. Hemodynamic monitoring provides valuable information about blood volume and cardiac function to guide fluid therapy.
Correction of Electrolyte and Acid-Base Imbalance
In cases of hyperkalemia, calcium gluconate (10%, 20 mL) can be administered intravenously at a slow rate or infused in glucose solution to counter the toxic effects of potassium on the heart. Sodium bicarbonate (5%, 100 mL) or a combination of glucose (25 g) and insulin (6 units) can also be infused intravenously to shift potassium into cells and lower serum potassium levels. When serum potassium levels exceed 6.5 mmol/L or hyperkalemia-pattern changes are observed on an electrocardiogram, emergency blood purification therapy becomes necessary. Mild metabolic acidosis does not require intervention, while sodium bicarbonate supplementation is given only if blood bicarbonate concentrations fall below 15 mmol/L.
Nutritional Support
Proper nutritional support minimizes protein catabolism, slows the rise in blood urea nitrogen and serum creatinine, promotes the repair and regeneration of damaged renal cells, and improves survival rates in ARF patients. Enteral nutrition is the preferred route of nutritional support if the patient's condition permits. Imbalances in the ratio of essential amino acids to non-essential amino acids should be considered in patients not undergoing renal replacement therapy.
Infection Control
Infection control is a critical measure to slow the progression of ARF. Infection sites should be managed actively, and preventive measures should be taken to avoid catheter-related infections. Antibiotic selection must avoid nephrotoxic drugs and potassium-containing formulations. Dosage and administration methods should be adjusted based on pharmacokinetics and pharmacodynamics.
Renal Replacement Therapy (RRT)
Also referred to as blood purification, RRT is a treatment method that uses artificial processes to replace renal functions, allowing for the removal of fluid and solutes from the body while correcting water, electrolyte, and acid-base imbalances. It is an essential therapeutic approach for kidney failure. Common methods include:
Hemodialysis (HD)
During hemodialysis, material exchange between blood and dialysate occurs across a semipermeable membrane. The primary mechanism of solute transport is diffusion. HD is highly efficient at removing small molecules, such as urea, creatinine, potassium, and sodium, but is less effective at clearing medium-sized molecules, such as inflammatory mediators.
Hemofiltration (HF)
HF uses pressure gradients across the filter membrane to remove water and solutes through ultrafiltration, with solute transport mechanisms primarily driven by convection and diffusion. HF is more effective in clearing medium- and large-sized molecules, making it particularly beneficial for treating systemic inflammatory response syndrome.
Continuous Renal Replacement Therapy (CRRT)
CRRT provides steady, slow, and isotonic removal of water and solutes, making it more physiologically consistent and causing minimal volume fluctuations. It is especially suitable for hemodynamically unstable patients. CRRT gradually lowers plasma osmolality to prevent disequilibrium syndrome, maintains water, electrolyte, and acid-base balance effectively, facilitates nutritional support, and clears medium- and large-sized molecules as well as inflammatory mediators. This improves outcomes in patients with severe infections and multiple organ dysfunction syndrome (MODS).
Peritoneal Dialysis
The advantages of peritoneal dialysis include simplicity of equipment and procedures, safety, and ease of implementation. It does not require vascular access or anticoagulation, making it particularly suitable for patients with bleeding tendencies, postoperative conditions, trauma, or intracranial hemorrhage. It also ensures stable hemodynamics and facilitates nutritional support.
Treatment During the Polyuric Phase
In early polyuric phase, glomerular filtration rate may remain impaired while tubular concentration functions are still inadequate, leading to continued elevations in serum creatinine, blood urea nitrogen, and potassium levels. As urine output increases significantly, water and electrolyte imbalances may arise. During this stage, patients often remain in poor general condition, with inadequate protein reserves and susceptibility to infections. Clinical vigilance in monitoring and treatment must continue. The main focus of treatment during this phase is maintaining water, electrolyte, and acid-base balance, controlling azotemia, addressing the primary disease, and preventing complications.
Prevention
Maintenance of Renal Perfusion Pressure
Close monitoring of a patient's hemodynamic changes is essential, along with maintaining adequate cardiac output, mean arterial pressure, and vascular volume to ensure renal perfusion and prevent renal ischemia.
Avoidance of Nephrotoxic Medications
Several factors require particular attention: (1) Patients who are elderly, have systemic infections, heart failure, liver cirrhosis, impaired renal function, hypovolemia, or hypoalbuminemia are especially sensitive to nephrotoxic drugs and demand careful consideration. (2) The nephrotoxicity of a drug is directly influenced by its dosage and blood concentration, necessitating the selection of appropriate doses and administration methods. (3) The simultaneous use of two or more nephrotoxic drugs should be avoided.
Infection Control
Controlling infections represents a key measure in preventing AKI. Efforts to identify infection sources, thoroughly eliminate infection sites, use antibiotics appropriately, and prevent catheter-associated and ventilator-associated iatrogenic infections contribute significantly to mitigating risk.
Removal of Nephrotoxic Substances
Effective fluid resuscitation plays a significant role in reducing myoglobinuria-induced nephrotoxicity, thus helping to prevent AKI.
Prevention of Contrast-Induced Nephropathy
This requires strict limitation of contrast agent dosage, the use of nonionic iso-osmolar contrast agents in high-risk patients, and intravenous administration of isotonic fluids to minimize the risk of contrast-induced nephropathy.