The intensive care unit (ICU) is a specialized ward unit dedicated to the centralized monitoring and treatment of critically ill patients. The ICU focuses on patients suffering from dysfunction of one or more organs and systems due to various causes, life-threatening conditions, or high-risk factors. Through the use of advanced diagnostic, monitoring, and treatment techniques, the ICU conducts continuous, dynamic, qualitative, and quantitative observation of the patient’s condition. Timely and effective interventions provide life-sustaining treatment and support to critically ill patients. The capability of organ function monitoring and treatment within an ICU directly reflects a hospital’s overall critical care ability and represents its comprehensive medical strength. It serves as an important measure of a modern hospital's institutional competency and infrastructure.
Primary Focus of Intensive Monitoring and Treatment
The ICU primarily focuses on using advanced monitoring and life support technologies to conduct continuous and dynamic qualitative and quantitative monitoring of patients’ physiological functions. This facilitates the assessment of pathophysiological states, disease severity, and the urgency of treatment, ultimately providing standardized, high-quality life support to improve treatment success rates.
Purposes of Monitoring
The purposes include:
- Early identification of high-risk factors that severely threaten patient survival, enabling prompt interventions.
- Continuous evaluation of organ functional status, serving as a basis for the prevention and treatment of organ dysfunction.
- Assessment of the severity of primary illnesses and prediction of disease progression and prognosis in critically ill patients.
- Guidance for diagnosis and differential diagnosis of diseases.
- Support for goal-directed treatment by providing physiological parameters obtained through continuous monitoring and evaluating their responses to therapy. This enables adjustments to treatment plans (such as intervention strategies, drug dosages, and infusion rates) in real-time to achieve target physiological indicators and reduce mortality rates significantly.
Key Areas of Intensive Monitoring and Treatment
Monitoring of critically ill patients now encompasses comprehensive bedside, real-time assessments of the entire body’s organ systems. ICU monitoring has evolved from monitoring basic vital signs to assessing full-scale organ system functions and even evaluating tissue metabolism levels.
Circulatory System
Electrocardiographic Monitoring
This is a standard monitoring modality used to evaluate the rate and rhythm of heartbeats, diagnose arrhythmia types, and assess myocardial ischemia.
Hemodynamic Monitoring
This involves both non-invasive and invasive techniques that provide real-time data on blood volume, cardiac function, and circulatory status. Accurate monitoring results depend on the appropriate selection of monitoring methods. Invasive hemodynamic monitoring includes techniques such as arterial blood pressure measurement, pulmonary artery catheterization (Swan-Ganz catheter), and pulse contour analysis for continuous cardiac output monitoring (PiCCO).
Classical pulmonary artery catheterization allows quantitative assessment of the workload on the left and right ventricles, as well as measurements of cardiac output, pulmonary artery wedge pressure (PAWP), and central venous pressure (CVP). These parameters are clinically valuable for assessing cardiac preload and the risk of pulmonary edema. However, PAWP and CVP values are influenced by several factors, including cardiac compliance, valvular function, intrathoracic pressure, and vascular tone, which makes guiding fluid therapy based solely on static PAWP and CVP measurements somewhat limited.
Dynamic hemodynamic parameters are therefore required in clinical practice to meet the monitoring needs of critically ill patients. Dynamic hemodynamic monitoring refers to assessing the heart's responsiveness to changes in specific preload indicators. Methods such as PiCCO monitoring and stroke volume variation (SVV) enable continuous dynamic tracking of parameters such as cardiac output, intrathoracic blood volume (ITBV), extravascular lung water (EVLW), and SVV. Among these, ITBV and SVV provide a more accurate reflection of the patient’s fluid responsiveness.
Non-invasive hemodynamic monitoring methods, such as passive leg-raising tests, bedside ultrasound, impedance-based analysis, and non-invasive cardiac output monitoring (NICO), are now routinely used to guide fluid management. These methods offer additional options for clinical hemodynamic monitoring, presenting non-invasive or minimally invasive alternatives for clinical decision-making.
Monitoring of Tissue Perfusion and Oxygenation Status
For surgical critically ill patients, tissue perfusion and oxygenation status are closely related to patient outcomes. Prolonged systemic hypoperfusion can result in irreversible organ damage.
Conventional Monitoring Indicators
Indicators such as blood pressure, pulse, urine output, and peripheral circulation status hold clinical significance for assessing the severity of shock and guiding fluid resuscitation. However, their inability to quantitatively evaluate tissue perfusion limits their application.
Venous-Arterial Carbon Dioxide Pressure Difference (Pcv-aCO2)
Pcv-aCO2, also referred to as the CO2 gap, is the difference in carbon dioxide partial pressure between the central vein and the artery, with a reference range of 2–5 mmHg. It is a reliable indicator of tissue perfusion adequacy. When systemic blood flow is sufficient, the microvascular circulation can efficiently clear CO2 produced by tissues, typically keeping Pcv-aCO2 below 6 mmHg. Insufficient systemic blood flow causes microcirculatory stasis, leading to greater CO2 diffusion into the microcirculation and an elevated Pcv-aCO2 (≥6 mmHg).
Blood Lactate Levels
Normal blood lactate levels are ≤2 mmol/L. Elevated lactate levels (>4 mmol/L) persisting for more than 48 hours indicate a poor prognosis, with mortality rates exceeding 80%. Lactate clearance rates provide a more accurate reflection of tissue perfusion and patient outcomes compared to absolute lactate values alone. In common surgical conditions such as hypovolemic shock and septic shock, normalizing blood lactate levels within the first 24 hours of resuscitation is critical. It is also important to note that blood lactate levels can be influenced by factors such as hepatic and renal function, the use of biguanide antidiabetic medications, and metabolic disorders, necessitating differentiation in clinical practice.
Mixed Venous Oxygen Saturation (SvO2)
SvO2, representing pulmonary artery oxygen saturation and central venous saturation, is a key parameter that reflects tissue oxygenation balance. Normal SvO2 ranges from 70% to 75%. Values below 60% indicate threats to systemic tissue oxygenation, while values below 50% signify severe tissue hypoxia. Central venous oxygen saturation (ScvO2), representing the oxygen saturation of blood in the superior vena cava or right atrium, has a reference range of 70–80% and correlates well with SvO2. It is widely used in clinical practice to assess overall tissue perfusion and oxygenation status.
Direct Visualization of Microcirculatory Perfusion
Technologies such as orthogonal polarization spectral imaging (OPS) allow direct visualization of microcirculation at the bedside and represent a recent advancement in microcirculation monitoring. These technologies enable assessments of tissue perfusion by calculating parameters such as perfused vessel density (PVD), proportion of perfused vessels (PPV), and microcirculatory flow index (MFI) through semi-quantitative analysis. Advantages of such methods include direct visualization, simplicity of operation, and non-invasiveness.
Respiratory System
Respiratory Function Monitoring
Acute respiratory failure is not uncommon in surgical patients, and pulmonary complications are a primary cause of respiratory failure and death in such patients. Individuals with abnormal preoperative lung function are more prone to pulmonary complications. Monitoring of lung ventilation and gas exchange functions is essential for evaluating the extent of lung damage and the effectiveness of respiratory therapy.
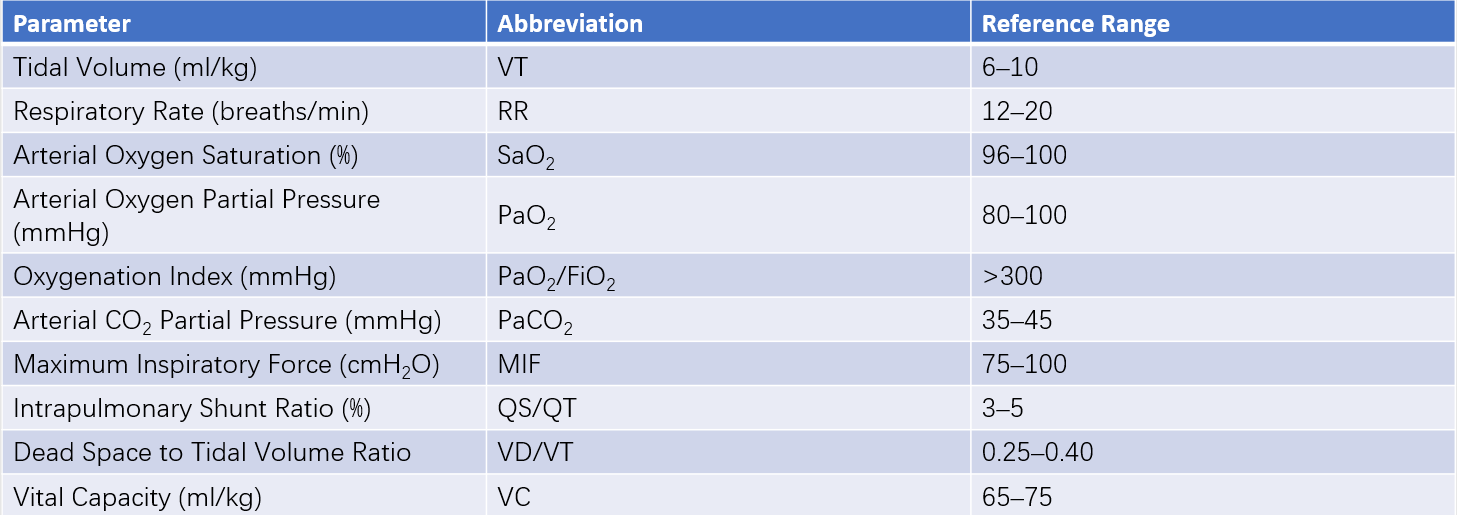
Table 1 Common parameters for respiratory function monitoring
Respiratory Therapy
Oxygen Therapy
Oxygen therapy involves the use of oxygen delivery systems or techniques to provide an inspired oxygen concentration (FiO2) greater than that of ambient air to correct hypoxemia. When alveolar gas exchange is intact, oxygen therapy facilitates oxygen diffusion from the alveoli into the bloodstream, thereby increasing arterial oxygen tension (PaO2). Oxygen therapy proves effective for mild ventilation impairment or pulmonary infections. However, in cases of collapsed alveoli, pulmonary edema, or reduced pulmonary blood perfusion causing ventilation-perfusion mismatch, oxygen therapy alone is less effective. Transitioning to mechanical ventilation and addressing the underlying condition becomes necessary.
Oxygen delivery methods include:
- High-Flow Systems: These systems deliver all the gas the patient breathes at a high flow rate. FiO2 is stable and adjustable. Common devices include Venturi masks or high-flow humidified oxygen therapy devices.
- Low-Flow Systems: These provide oxygen flow rates lower than the patient’s total inspiratory demand, requiring supplemental air intake. FiO2 is therefore unstable and less controllable. Common methods include nasal cannulas, simple face masks, and reservoir bag masks.
Mechanical Ventilation
Mechanical ventilation refers to the process of generating a pressure gradient between the airway and alveoli through a ventilator, facilitating the flow of gas into the alveoli. It is an effective treatment for respiratory failure, with current clinical practice primarily utilizing positive-pressure ventilation. The objectives of mechanical ventilation include improving and maintaining pulmonary ventilation and gas exchange, reducing the workload of respiratory muscles, and meeting specific therapeutic needs, such as treating flail chest. However, mechanical ventilation can also induce or exacerbate lung injuries, collectively referred to as ventilator-induced lung injury (VILI), which includes barotrauma, volutrauma, and biotrauma.
Common modes of mechanical ventilation include:
A. Controlled Mechanical Ventilation (CMV)
The ventilator delivers mechanical ventilation to the patient according to pre-set parameters, with the patient breathing passively. This mode is typically applied in patients without spontaneous breathing for various reasons.
B. Assist-Control Ventilation (A/CV)
The ventilator synchronizes with the patient's spontaneous breathing, delivering a pre-set tidal volume. This synchronization is triggered by negative pressure or airflow generated during the patient's inspiration, and the trigger threshold can be adjusted. To prevent hypoventilation caused by an excessively low spontaneous breathing rate, a backup respiratory rate is set. If the interval between the patient's breaths exceeds the backup interval, the ventilator initiates controlled breaths.
C. Synchronized Intermittent Mandatory Ventilation (SIMV)
This mode combines mandatory positive-pressure ventilation with spontaneous breathing, allowing patients to breathe spontaneously during mechanical ventilation. The breathing frequency can be controlled by the patient, while the ventilator delivers breaths at a fixed rate. Each positive-pressure breath is triggered by the patient’s inspiratory effort.
D. Pressure Support Ventilation (PSV)
This mode is suitable only for patients with spontaneous breathing, aiming to reduce the work of breathing. At the start of inspiration, the ventilator delivers air, rapidly increasing airway pressure to a pre-set level. When the inspiratory flow falls below a certain threshold, the ventilator switches to the expiratory phase.
E. Positive End-Expiratory Pressure (PEEP)
During mechanical ventilation, the airway pressure at the end of expiration is maintained above atmospheric pressure through mechanical means. PEEP increases lung volume and functional residual capacity (FRC), prevents atelectasis, promotes the re-expansion of collapsed alveoli, and improves pulmonary compliance. This reduces intrapulmonary shunting and corrects hypoxemia. PEEP is commonly used in cases involving early small airway closure, atelectasis, or increased intrapulmonary shunting.
Patient Condition Assessment
Proper assessment of a patient's condition and prognosis is crucial in ICU management and treatment. Standardized evaluation methods carry several important implications:
- Accurate evaluation of the severity of the condition and prognosis;
- Rational selection of therapeutic interventions and medications, along with the assessment of their effectiveness;
- Provision of objective criteria for patient admission to or discharge from the ICU;
- Assessment of the quality of care based on the effectiveness of interventions.
Scoring systems for critically ill patients provide quantitative and objective parameters for clinical evaluation. Common scoring systems include the following:
Acute Physiology and Chronic Health Evaluation II (APACHE II)
Among the APACHE scoring systems, APACHE II is the most commonly used. It evaluates a patient’s condition based on three components: acute physiological changes, chronic health conditions, and age. It includes 12 physiological parameters and the Glasgow Coma Scale, along with age and pre-existing health conditions, to provide a comprehensive assessment of disease severity. The APACHE II score ranges from a minimum of 0 to a maximum of 71, with higher scores indicating greater severity and worse prognosis. Generally, scores between 9–15 represent mild risk, 16–20 moderate risk, and scores above 20 indicate severe risk.
Therapeutic Intervention Scoring System (TISS)
Established in 1974, TISS evaluates the severity of a patient's condition based on the number and complexity of monitoring, treatment, care, and diagnostic measures undertaken. Higher TISS scores reflect more severe conditions and the greater number of interventions required. This system also assists in organizing healthcare resources and personnel. TISS scores above 40 indicate high-risk patients. While TISS is straightforward and easy to use, its limitations include a lack of consideration for the patient's age and pre-existing health conditions, as well as variations in monitoring and treatment practices among healthcare facilities.
Multiple Organ Dysfunction Score (MODS)
Proposed by Marshall in 1995 and modified by Richard in 2001, the MODS evaluates multi-organ dysfunction. Its features include a small number of parameters, simplicity, ease of use, and relatively accurate predictions of mortality and prognosis. The main limitation of MODS is its focus on the function of six common organ systems, without accounting for other prognostic factors.
Sepsis-Related Organ Failure Assessment (SOFA)
SOFA emphasizes early and dynamic monitoring. It evaluates six organ systems, with each system scored on a scale of 0–4 based on the most severe value recorded each day. SOFA has significant utility in diagnosing and assessing sepsis. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, with a diagnosis requiring infection and a SOFA score of ≥2.
Humanistic Care in Intensive Monitoring and Treatment
Surgical critically ill patients in the ICU often experience heightened stress, which commonly arises from the following factors:
Impact of Severe Illness
This includes the inability to care for oneself due to serious illness, the need to endure various invasive diagnostic and therapeutic procedures, and the pain caused by one’s injuries or disease.
Environmental Factors
Factors such as being confined to the bed, prolonged exposure to artificial lighting, persistent noises (e.g., sounds from machines and alarms), and sleep deprivation contribute significantly to patient stress.
Pain and Discomfort
Sources of pain and discomfort include surgical wound pain, discomfort associated with endotracheal intubation and other types of catheterization, and prolonged bedrest.
Anxiety About Personal Condition
Concerns regarding one’s own health status further contribute to emotional and psychological stress in ICU settings. ICU healthcare professionals are expected to adopt individualized and compassionate care strategies tailored to the patient's condition, disease characteristics, and specific needs, with the goal of reducing stress and minimizing its adverse effects.
Effective pain and sedation management in ICU patients helps alleviate or eliminate pain and physical discomfort, reducing adverse stimuli and excessive activation of the sympathetic nervous system. Such management improves sleep quality, induces amnesia, and diminishes or eliminates patients' memories of pain and trauma during ICU treatment. It also helps to reduce or eliminate anxiety, agitation, and delirium, preventing unconscious patient behaviors that might interfere with treatment. Furthermore, it lowers the stress burden on organs, preserving organ reserve function.
For these reasons, pain and sedation management have become integral components of humanistic care in the ICU. In addition, the therapeutic process emphasizes safeguarding patient privacy, respecting patient rights, enhancing patient and family education about the condition, and improving communication strategies with patients and their families. Through various humanistic care measures, the aim is to mitigate the painful experiences of critically ill patients during intensive monitoring, lower their physical discomfort and psychological stress, and ultimately support recovery from illness.