The spinal canal contains two spaces that can be utilized for anesthesia: the subarachnoid space and the epidural space. Based on the site where local anesthetics are administered, spinal anesthesia is divided into subarachnoid anesthesia (spinal anesthesia), epidural anesthesia, caudal anesthesia, and combined spinal-epidural anesthesia (CSE). These methods are collectively referred to as neuraxial anesthesia.
Anatomical Basis
Spine and Spinal Canal
The spine is composed of stacked vertebrae. A single vertebra consists of the vertebral body in the front and the vertebral arch at the back, with the vertebral foramen located in between. The alignment of all vertebral foramina forms the spinal canal. The spinal canal starts at the foramen magnum of the occipital bone and ends at the sacral hiatus. The spine exhibits four physiological curves: cervical, thoracic, lumbar, and sacral curves. When a patient is in the supine position, the highest points are located at C3 and L3, while the lowest points are at T5 and S4. These characteristics significantly impact the distribution of local anesthetics during lumbar anesthesia.
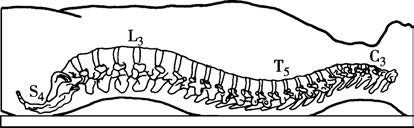
Figure 1 Physiological curvature of the spine
Ligaments
From the outside inwards, the ligaments include the supraspinous ligament, interspinous ligament, and ligamentum flavum. The supraspinous ligament connects the tips of the spinous processes and is relatively tough, often undergoing calcification in elderly patients. The interspinous ligament connects adjacent spinous processes and has a looser structure. The ligamentum flavum connects the adjacent laminae and covers the interlaminar foramen. It is almost entirely composed of elastic fibers and exhibits a noticeable resistance during needle insertion. In spinal canal anesthesia, the needle traverses the skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, and ligamentum flavum to reach the epidural space. If the needle passes further through the dura mater and arachnoid mater, it enters the subarachnoid space.
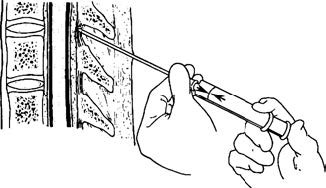
Figure 2 Ligamentous elasticity of the ligamentum flavum
Spinal Cord, Spinal Membranes, and Spaces
The spinal canal contains the spinal cord and three layers of spinal membranes. The lower end of the spinal cord in adults usually terminates at the inferior border of the L1 vertebral body or the superior border of L2. In newborns, it ends at the inferior border of L3 and gradually ascends with age. Therefore, in adults, lumbar punctures for spinal anesthesia should be performed below L2, while in children, they should be conducted below L3.
The spinal membranes are, from the inside outwards, the pia mater, arachnoid mater, and dura mater. The dura mater is formed by dense connective tissue, has limited blood supply, and heals slowly if punctured. The space between the pia mater and arachnoid mater is called the subarachnoid space, which is contiguous with the cranial subarachnoid space at its superior end and terminates at the S2 level at its inferior end. The subarachnoid space contains cerebrospinal fluid. At the S2 level, the dura mater and arachnoid mater form a closed dural sac. The epidural space lies between the dura mater and the inner wall of the spinal canal (consisting of the ligamentum flavum and periosteum) and contains fat, loose connective tissue, blood vessels, and lymphatic vessels. The epidural space closes at the foramen magnum and terminates at the sacral hiatus. A potential space, the subdural space, exists between the dura mater and arachnoid mater.
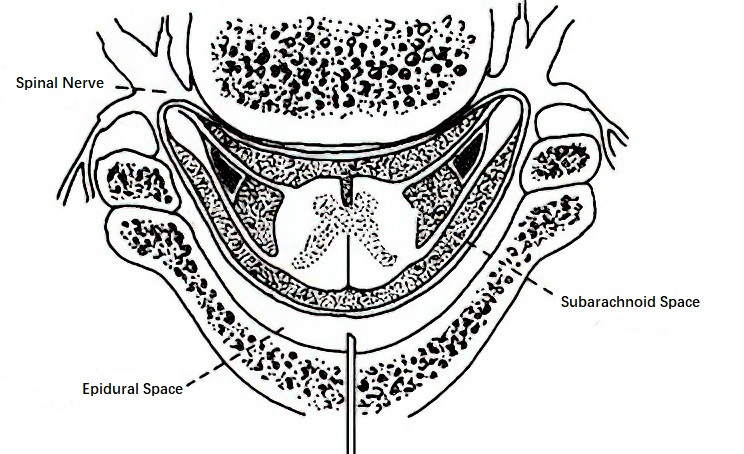
Figure 3 Cross-section of the spinal canal
The three layers of spinal membranes extend laterally to envelop the spinal nerve roots, forming the root dura mater, root arachnoid mater, and root pia mater. These structures play a role in the diffusion of local anesthetics within the spinal canal.
Sacral Canal
The sacral canal is the spinal canal space within the sacrum. Anesthesia administered by injecting local anesthetics into this space is referred to as caudal anesthesia, which is a type of epidural anesthesia. The sacral canal contains loose connective tissue, fat, and a rich venous plexus, with a volume of approximately 25–30 mL. Since the dural sac terminates at the S2 level, the sacral canal is considered part of the epidural space. The sacral canal ends at the sacral hiatus, which has a V- or U-shaped opening covered by the sacrococcygeal ligament. On either side of the hiatus are small, bony protrusions known as sacral cornua, which serve as important anatomical landmarks for locating the puncture site during caudal anesthesia. The average distance from the dural sac to the sacral hiatus is 47 mm. To avoid accidental entry into the subarachnoid space, the depth of the needle insertion should be carefully controlled during caudal punctures.
Spinal Nerves
There are 31 pairs of spinal nerves: 8 cervical nerves (C), 12 thoracic nerves (T), 5 lumbar nerves (L), 5 sacral nerves (S), and 1 coccygeal nerve (Co). Each spinal nerve is formed by the merging of an anterior (ventral) root and a posterior (dorsal) root. The ventral root arises from the anterior horn of the spinal cord and consists of motor nerve fibers and sympathetic efferent fibers (as well as parasympathetic efferent fibers in the sacral region). The dorsal root contains sensory nerve fibers and sympathetic afferent fibers (and parasympathetic afferent fibers in the sacral region), which enter the posterior horn of the spinal cord. The thickest fibers are motor fibers, followed by sensory fibers, with the thinnest being sympathetic and parasympathetic fibers.
Mechanisms and Physiological Effects
Cerebrospinal Fluid
The total volume of cerebrospinal fluid (CSF) in adults is approximately 120–150 mL, with only 25–30 mL located within the subarachnoid space of the spine. It is clear and transparent, with a pH of 7.35 and a specific gravity of 1.003–1.009. The CSF pressure in the lateral decubitus position is 0.69–1.67 kPa (70–170 mmH2O), increasing to 1.96–2.94 kPa (200–300 mmH2O) in the seated position. During spinal anesthesia, CSF can dilute the local anesthetic.
Site of Drug Action
In spinal anesthesia, the local anesthetic acts directly on the spinal nerve roots and the surface of the spinal cord. In epidural anesthesia, the mechanisms by which the local anesthetic takes effect may include:
- Passage through the arachnoid villi into the subarachnoid space at the nerve roots, affecting the spinal nerve roots.
- Diffusion through the intervertebral foramen, leading to paravertebral blockade of the spinal nerves. Since the neural sheath at the intervertebral foramen is thin, the local anesthetic may penetrate this site to affect the spinal nerve roots.
- Direct penetration through the dura mater and arachnoid mater into the subarachnoid space, where the local anesthetic acts on the spinal nerve roots and spinal cord surface similarly to spinal anesthesia.
The primary site of action for spinal canal anesthesia is the spinal nerve roots. Since the subarachnoid space contains CSF, the injected local anesthetic becomes diluted, and the exposed spinal nerve roots are easily blocked. Therefore, compared to epidural anesthesia, spinal anesthesia employs higher concentration, smaller volume, and lower doses of medication (approximately 1/5 to 1/4 of the dose required for epidural anesthesia), while the diluted concentration is significantly lower than that used in epidural anesthesia.
Anesthetic Plane and Blocking Effects
The anesthetic plane refers to the range where pain sensation has been eliminated, as determined by pinprick testing. Blocking of the sympathetic nerves reduces visceral traction responses, sensory nerve blockade prevents pain transmission, and motor nerve blockade induces muscle relaxation. Due to differences in nerve fiber diameters, sympathetic nerves are blocked first, with the block plane generally being 2–4 segments higher than that of the sensory nerves. Motor nerves are blocked last, and their plane is 1–4 segments lower than that of sensory nerves. The distribution of spinal nerve segments on the body's surface is illustrated.
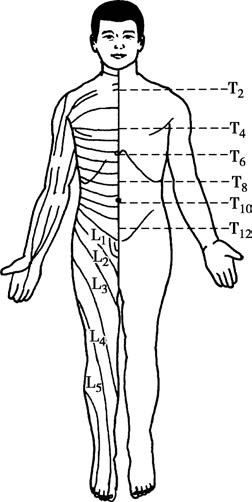
Figure 4 Segmental distribution of spinal nerves on the body surface
By referencing anatomical landmarks on the body's surface, different nerves are associated with:
- T2 at the level of the sternal angle,
- T4 at the nipple line,
- T6 below the xiphoid process,
- T8 at the subcostal margin,
- T10 at the level of the umbilicus,
- T12 approximately 2–3 cm above the pubic symphysis,
- L1–3 at the anterior thigh,
- L4–5 at the anterior lower leg and dorsum of the foot,
- S1–5 at the posterior thigh, lower leg, and the perianal region.
For instance, if the upper boundary for sensory loss is at the nipple line (T4) and the lower boundary is at the umbilical line (T10), the anesthetic plane is described as T4–T10.
Physiological Effects
Effects on Respiration
The impact on respiration depends on the height of the block plane, with motor nerve blockade being particularly significant. If the thoracic spinal nerves are blocked, most or all of the intercostal muscles may become paralyzed, reducing or eliminating thoracic respiration. However, as long as the phrenic nerve (C3–5) remains unblocked, basic pulmonary ventilation can be maintained. If the diaphragm is also paralyzed, abdominal breathing may diminish or cease, which could cause hypoventilation or even respiratory arrest. Reducing the concentration of local anesthetic during high-level epidural anesthesia and monitoring respiration and upper limb muscle strength can help mitigate respiratory suppression and guide timely intervention.
Effects on Circulation
Hypotension
Spinal canal anesthesia blocks the sympathetic nerves, causing vasodilation of small arteries, reduced peripheral resistance, and venous dilation, which increases blood volume in the venous system and decreases venous return to the heart. This leads to reduced cardiac output and hypotension. The occurrence and degree of hypotension depend on the height of the anesthetic plane as well as the patient’s overall condition. Particular attention should be paid to patients with insufficient preoperative preparation, existing hypovolemia, atherosclerosis, heart failure, high anesthetic planes, or extensive nerve blockages.
Bradycardia
Bradycardia may occur due to enhanced vagal tone after sympathetic nerve blockage. In high-level blocks, blockade of the cardiac accelerator nerves (T4 and above) can also slow the heart rate.
Effects on Other Systems
During spinal canal anesthesia, enhanced vagal activity may increase gastrointestinal peristalsis, leading to nausea and vomiting. The procedure may also impact liver and kidney function, and urinary retention could occur in some patients.
Methods
Subarachnoid Anesthesia
The process of injecting local anesthetic into the subarachnoid space to block the conduction of certain spinal nerves, thereby inducing anesthesia in the corresponding innervated region, is referred to as subarachnoid anesthesia, also known as spinal anesthesia.
Classification
Classification is based on the method of administration, anesthetic plane, and the specific gravity of the local anesthetic solution.
- By administration method: Subarachnoid anesthesia can be divided into single injection and continuous injection.
- By anesthetic plane: A block plane at or below T10 is categorized as a low plane; above T10 but below T4 is considered a medium plane; T4 or higher constitutes a high plane spinal anesthesia, although this is no longer used in clinical practice.
- By specific gravity of the local anesthetic solution: Local anesthetic solutions may be formulated to have lower, equal, or higher specific gravity relative to cerebrospinal fluid. By using the principle of light solutions rising and heavy solutions sinking, positioning can adjust the anesthetic plane.
Spinal Puncture
Patients are typically placed in the lateral decubitus position (sitting position can be used for saddle anesthesia), with the hips and knees flexed, the head and neck flexed toward the chest, and the lumbar spine maximally arched to widen the interspinous spaces for easier puncture. The puncture site in adults is usually chosen at the L3–4 interspace, avoiding puncture at or above L1–2. The highest points of the iliac crests are palpated, and a line connecting these points intersects the spine at the L4 spinous process or the L3–4 interspace.
For a midline approach, local anesthetic is used to create a wheal in the midline of the skin, and deeper layers up to the interspinous ligament are infiltrated. The needle is inserted perpendicular to the patient’s back with the bevel parallel to the dura mater fibers. Resistance is often felt when penetrating the ligamentum flavum, followed by a distinct loss of resistance when entering the epidural space, and then another loss of resistance sensation as the needle passes through the dura mater. Cerebrospinal fluid dripping from the needle confirms successful puncture. The prepared local anesthetic can then be injected via syringe.
For the paramedian approach, the needle is inserted 1–1.5 cm lateral to the midline at an angle of approximately 75° to the skin, targeting the subarachnoid space while avoiding the calcified supraspinous ligament. This technique is suitable for elderly patients with ligament calcification, obese individuals, or cases where midline puncture is difficult.
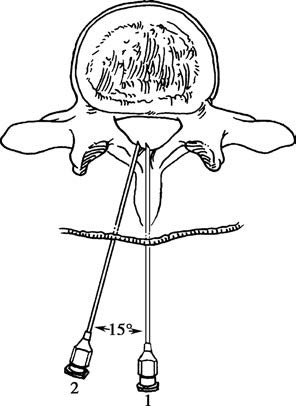
Figure 5 Midline approach and paramedian approach
Commonly Used Medications for Spinal Anesthesia
Procaine
The dosage for adults is 100–150 mg, or 50–100 mg for saddle anesthesia. A 5% hyperbaric procaine solution is prepared by dissolving 150 mg of procaine in 3 mL of 5% glucose or cerebrospinal fluid. The duration of action is 1–1.5 hours.
Tetracaine
The adult dosage is 8–15 mg. A 0.33% hyperbaric tetracaine solution can be prepared by mixing 1 mL of 1% tetracaine (10 mg) with 1 mL each of 10% glucose and 3% ephedrine in a 1:1:1 ratio. The onset time is 5–10 minutes, and the duration of action is 2–2.5 hours.
Bupivacaine
The typical dose is 10–15 mg. A 0.33%–0.5% hyperbaric solution can be made by mixing 2 mL of 0.5%–0.75% bupivacaine with 1 mL of 10% glucose. It has a faster onset compared to tetracaine, with a duration of 1.5–3.3 hours.
Ropivacaine
The usage is similar to that of bupivacaine.
Replacing glucose in these formulations with sterile water for injection produces hypobaric solutions.
Adjustment of Anesthetic Plane
After injection, the anesthetic plane should be adjusted and controlled within 5–10 minutes. It becomes challenging to make adjustments once the drug binds to neural tissue. If the anesthetic plane is too low to meet surgical requirements, a different anesthesia method may be necessary. Overly high planes can cause significant physiological effects and may even endanger the patient’s life. Factors affecting the anesthetic plane include the specific gravity, dose, and volume of the anesthetic solution, the patient's height, spinal curvature, intraabdominal pressure, and other variables. However, the anesthetic dose is the primary determinant, with higher doses correlating with higher planes. If these factors remain constant, the puncture site, patient positioning, and injection speed become critical factors for adjustment.
Puncture Site
Puncture at the L2–3 interspace with a hyperbaric anesthetic solution may allow the drug to flow upward along the thoracic spinal curvature when the patient is moved to a supine position, potentially resulting in a higher anesthetic plane. Puncture at the L4–5 interspace generally leads to a lower anesthetic plane.
Patient Positioning
After drug administration, positioning the patient in a supine position within 5–10 minutes enables adjustment of the anesthetic plane. If a hyperbaric solution is used and the plane is too low, the head of the operating table can be lowered to elevate the anesthetic plane. Once the desired plane is achieved, the table is returned to a horizontal position while monitoring the patient’s respiratory and circulatory status. For surgery on a single lower limb, the lateral decubitus position with the affected side down can be maintained for 5–10 minutes after injection to concentrate the anesthetic effect on the affected limb. Saddle anesthesia, for procedures involving the anus or perineum, is performed at the L4–5 interspace.
Injection Speed
Faster injection speeds result in broader anesthetic coverage, while slower speeds produce more localized effects. A typical injection speed is 1 mL per 5 seconds.
Indications and Contraindications
Spinal anesthesia is suitable for lower abdominal, pelvic, lower limb, and perineal surgeries lasting 2–3 hours, such as cesarean sections, appendectomies, hernia repairs, meniscectomies, hemorrhoidectomies, and anal fistula excisions.
Contraindications include:
- Central nervous system diseases such as meningitis, poliomyelitis affecting the anterior horn of the spinal cord, and others.
- Coagulopathy.
- Shock.
- Infection at the puncture site.
- Sepsis.
- Spinal injury or tuberculosis.
- Acute heart failure or myocardial infarction.
- Demyelinating neurological disorders such as nerve damage from burns, electrocution, paraplegia, or polyneuropathy.
In elderly patients, as well as those with cardiovascular disease or hypertension, the dosage and anesthetic plane should be strictly controlled. Patients unable to cooperate, such as those with psychiatric disorders, are not candidates for spinal anesthesia.
Epidural Anesthesia
The method of anesthesia involving the injection of local anesthetic into the epidural space to block the conduction of certain spinal nerves, resulting in the loss of sensation and/or motor function in their innervated regions, is known as epidural anesthesia. This technique is also referred to as epidural space anesthesia. It can be performed using a single injection or a continuous method, with the continuous method being more commonly used in clinical practice.
Epidural Puncture Technique
Epidural puncture can be performed at the cervical, thoracic, lumbar, or sacral levels. As there is no cerebrospinal fluid in the epidural space, the injected anesthetic spreads in both directions depending on its volume. For this reason, the central point of the surgical area is generally chosen as the interspinous space for puncture. The patient's position during the procedure, the puncture method, and the layers traversed during puncture are largely similar to those of spinal anesthesia. Unlike spinal anesthesia, however, the needle only passes through the ligamentum flavum and does not puncture the dura mater. This results in only one "loss of resistance" sensation.
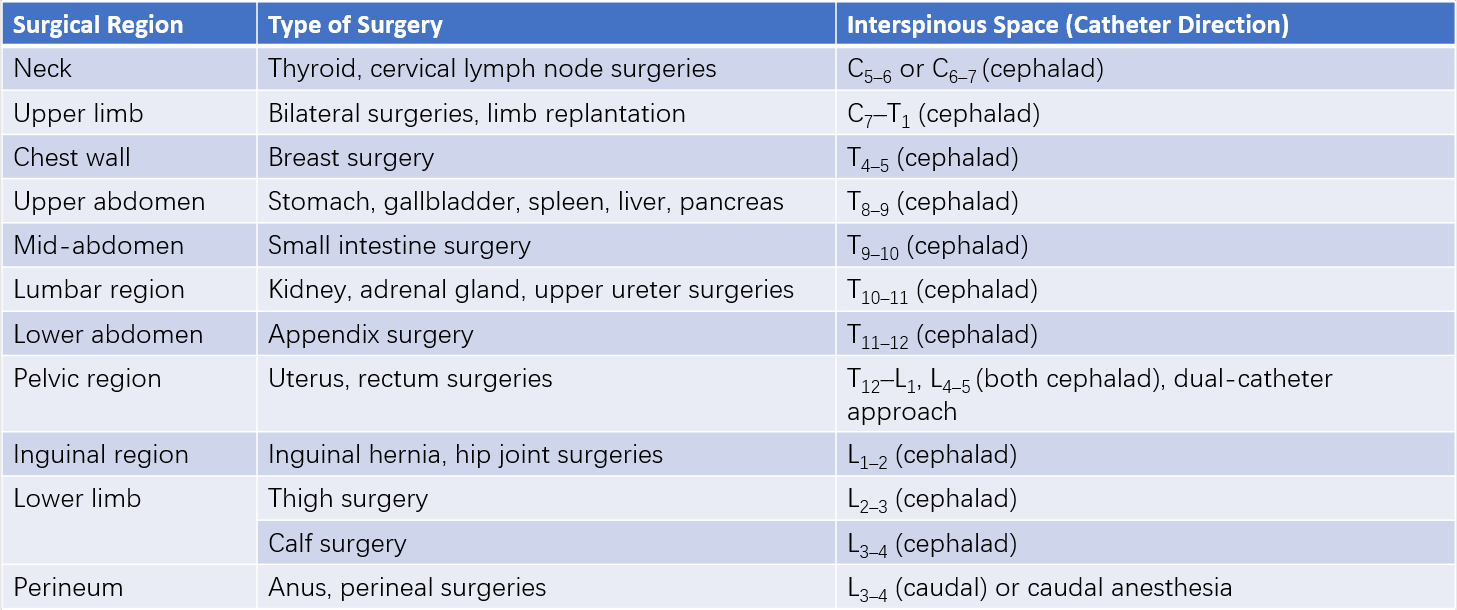
Table 1 Selection of interspinous spaces for epidural puncture based on surgical site
The method for confirming that the needle has reached the epidural space includes the following:
- Loss-of-resistance method: When the needle passes through the ligamentum flavum, a loss of resistance is felt. The injection of liquid encounters no resistance, and low-resistance syringes confirm this decrease in resistance. The absence of cerebrospinal fluid upon aspiration further indicates that the needle tip has reached the epidural space.
- Capillary negative pressure method: After reaching the ligamentum flavum, the puncture needle is connected to a glass capillary containing liquid. As the needle advances slowly, a loss-of-resistance sensation is felt, and the liquid in the tube is drawn inward due to the negative pressure characteristic of the epidural space, confirming correct placement.
Afterward, a catheter is inserted into the epidural space to a depth of approximately 3–4 cm. The needle is withdrawn, the catheter is secured in place, and an injection device is attached.
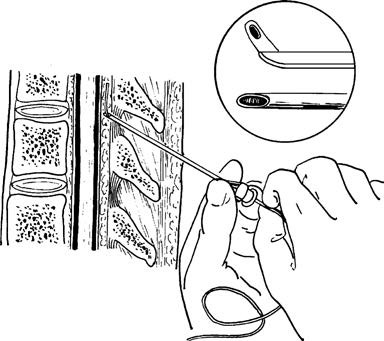
Figure 6 Illustration of epidural catheter insertion into the epidural space via a puncture needle
The upper right inset shows an enlarged view of the beveled opening at the tip of the epidural puncture needle and the passage of the epidural catheter through the beveled opening.
Common Local Anesthetics and Injection Methods
Common anesthetics include:
- Lidocaine (1.5%–2%): Onset time is 5–8 minutes, with a duration of approximately 1 hour.
- Tetracaine (0.25%–0.33%): Onset time is 10–20 minutes, with a duration of 1.5–2 hours.
- Bupivacaine (0.5%–0.75%): Onset time is 7–10 minutes, with a duration of 2–3 hours.
- Ropivacaine (0.75%): This is also frequently used.
After successful catheter placement, a test dose of 3–5 mL of 2% lidocaine is injected, and the patient's response is observed for 5–10 minutes. If no signs indicating spinal anesthesia are observed, such as a rapidly appearing anesthetic block or lower limb motor impairment, additional doses are determined based on the test dose's effects and the patient's overall condition. The sum of the test dose and the supplemental doses is referred to as the initial dose. When the effect of the initial dose begins to wane, a second dose is administered, which typically amounts to half or two-thirds of the initial dose.
If the epidural catheter is unintentionally placed in the subarachnoid space, a "transected" anesthetic plane, along with pronounced lower limb motor impairment and a drop in blood pressure, may appear within 5 minutes of the test dose. In such cases, the administration of further anesthetic should cease immediately. Emergency measures should be undertaken in cases of severe hypotension or respiratory difficulty.
Adjustment of the Anesthetic Plane
The anesthetic plane in epidural anesthesia differs from spinal anesthesia, exhibiting a segmental distribution. The main factors influencing the anesthetic plane include:
- Volume of local anesthetic injected: The spread of the drug within the epidural space is determined by its volume. Greater volumes result in wider diffusion and larger anesthetic coverage.
- Puncture site: The height of the upper and lower anesthetic planes depends on the puncture site. An improperly chosen site can result in an anesthetic plane that is either too high, risking respiratory and circulatory suppression, or too low, which may fail to meet surgical requirements and lead to anesthetic failure.
- Catheter direction: If the catheter is inserted toward the head, the anesthetic tends to spread to the thoracic and cervical regions. If inserted caudally, the spread occurs toward the lumbar and sacral regions.
- Injection method: For the same total dose, a single injection produces a broader anesthetic plane, whereas dividing the dose into multiple injections narrows the plane. Drugs injected at the cervical level tend to diffuse more widely than those at the thoracic level, whereas thoracic injections have a broader range than lumbar ones.
- Patient factors: In elderly patients or those with conditions such as arteriosclerosis, pregnancy, dehydration, or frailty, the anesthetic plane is broader than usual, necessitating adjustment of the anesthetic dose.
- Other factors, such as the drug concentration, injection speed, and patient positioning, may also exert secondary effects.
Indications and Contraindications
Epidural anesthesia is commonly used for abdominal, lumbar, lower limb, and thoracic surgeries performed below the diaphragm. Unlike spinal anesthesia, the duration of surgery is not a restriction with this method. However, due to complexity in technique and risk management, epidural anesthesia is no longer routinely used for cervical or upper limb surgeries.
Contraindications are similar to those for spinal anesthesia. In elderly individuals, as well as patients with pregnancy, anemia, hypertension, cardiac disease, or hypovolemia, the use of epidural anesthesia requires caution. Medication dosage should be reduced, and close monitoring is essential.
Caudal Anesthesia
Caudal anesthesia is a type of epidural anesthesia in which local anesthetic is injected into the sacral canal through the sacral hiatus to block the sacral spinal nerves. It is primarily used for surgeries involving the rectum, anus, and perineal region.
Caudal Puncture Technique
Patients are positioned either in the lateral or prone position. For the lateral position, the lumbar spine is curved backward, and both knees are brought toward the abdomen. In the prone position, a small pillow is placed under the hips, the legs are slightly apart, the toes are inclined inward, and the heels are turned outward to relax the gluteal muscles.
Before puncture, the tip of the coccyx is palpated, and moving approximately 3–4 cm upward along the midline leads to a V- or U-shaped depression flanked by two bony prominences called the sacral cornua. This area is the location of the sacral hiatus.
A wheal is made at the center of the sacral hiatus, and the needle is inserted perpendicular to the skin through the sacrococcygeal ligament covering the sacral hiatus. A sudden loss of resistance is encountered upon piercing the ligament. At this point, the needle is angled at approximately 30° relative to the skin. If the needle hits bone, the angle is adjusted to align with the longitudinal axis of the sacral canal. Care is taken not to insert the needle beyond the horizontal level of the posterior superior iliac spine (S2) to avoid accidentally entering the subarachnoid space.
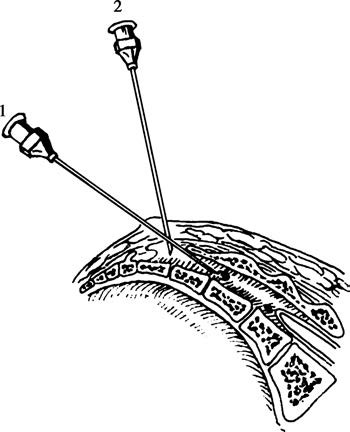
Figure 7 Caudal puncture technique
When using the simplified vertical puncture method, the patient is in the lateral position, and a short No. 7 needle is inserted vertically through the upper portion of the sacral hiatus to pierce the sacrococcygeal ligament. This approach is considered safer. Successful puncture is confirmed by connecting a syringe and ensuring that neither blood nor cerebrospinal fluid is aspirated. The local anesthetic is then injected without resistance, and no subcutaneous bulging occurs after injection.
Common Local Anesthetics
Caudal anesthesia commonly employs 1.5% lidocaine or 0.5% bupivacaine, with a typical adult dose of 20 mL. The duration of anesthesia is approximately 1.5–2 hours for lidocaine and 4–6 hours for bupivacaine. The drug is administered in divided doses, beginning with a test dose of 5 mL. After observing for 5 minutes without adverse reactions, the remaining 15 mL is injected.
Combined Spinal-Epidural Anesthesia
Combined spinal-epidural anesthesia (CSEA) is widely used for lower abdominal and lower limb surgeries. This method integrates the rapid onset, effective analgesia, and muscle relaxation of spinal anesthesia with the adjustable anesthetic plane and suitability for prolonged surgeries offered by epidural anesthesia.
Puncture Techniques
There are two approaches to CSEA:
Two-Puncture Approach
For this method, the patient is positioned as for spinal anesthesia. An epidural puncture is first performed at the T12–L1 interspace, and the catheter is placed. A subsequent spinal puncture is performed at the L3–4 or L4–5 interspace to access the subarachnoid space.
Single-Puncture Approach
This approach involves the use of a specially designed combined spinal-epidural puncture needle. The epidural space is accessed through the L2–3 interspace. After confirming successful epidural puncture, a matched 25G spinal needle is passed through the epidural needle to perform a spinal puncture. The appearance of cerebrospinal fluid confirms the subarachnoid space has been accessed, and the local anesthetic is injected for spinal anesthesia. After this, the spinal needle is withdrawn, and the epidural catheter is advanced toward the head and secured in preparation for use.
The spinal needle used in the single-puncture approach is non-cutting and conical, which minimizes damage to the dura mater during puncture and significantly reduces the incidence of post-operative headaches. However, the injection time for spinal anesthesia may take 45–60 seconds. This approach is the more commonly used method in clinical practice.
Complications and Management
Complications and Management of Spinal Anesthesia
Intraoperative Complications
Hypotension and Bradycardia
The higher the anesthetic plane and the broader the block range, the greater the degree of vascular dilation and the smaller the range of compensatory vasoconstriction, resulting in more significant hypotension. Low-planed spinal anesthesia generally causes less hypotension. Patients with hypertension or hypovolemia have reduced compensatory capacity and are at greater risk of developing hypotension. When the anesthetic plane reaches above T4, sympathetic nerves may be blocked, leading to relative vagal overactivity and causing bradycardia. In cases of significant hypotension, rapid intravenous infusion of 200–300 mL fluids may help expand blood volume, with intravenous administration of norepinephrine being necessary in some instances. Administration of atropine may address bradycardia.
Respiratory Depression
Respiratory depression frequently occurs in patients with high-level spinal anesthesia due to extensive blockade of thoracic spinal nerves, paralysis of intercostal muscles, and symptoms such as chest tightness, shortness of breath, difficulty speaking, weakened thoracic respiration, and cyanosis. Total spinal anesthesia, involving complete blockade of all spinal nerves, may result in respiratory arrest, hypotension, or cardiac arrest. Furthermore, excessively high anesthetic planes can cause ischemia and hypoxia in the respiratory center, worsening respiratory depression. Oxygen therapy and assisted ventilation via a face mask are indicated in cases of respiratory insufficiency. Endotracheal intubation and mechanical ventilation are required in the event of respiratory arrest.
Nausea and Vomiting
These symptoms are common in the following scenarios:
- An excessively high anesthetic plane causing hypotension and respiratory depression, leading to cerebral ischemia and hypoxia that stimulate the vomiting center.
- Enhanced vagal activity increasing gastrointestinal peristalsis.
- Traction on abdominal organs.
- Increased sensitivity to intraoperative medications.
Management should address the underlying causes, including oxygen therapy, raising blood pressure, administrating atropine before anesthesia, and temporarily halting surgical traction. Medications such as droperidol or ondansetron may also help prevent and treat this condition.
Postoperative Complications
Post-Dural Puncture Headache (PDPH)
Previously referred to as post-spinal headache, PDPH occurs at a rate of 3%–30% and generally begins 2–7 days after anesthesia, with young women being more commonly affected. The headache typically intensifies upon standing or sitting upright and alleviates or disappears when lying flat. Symptoms may resolve within four days for about half of the patients, with most cases resolving within a week, though some may last longer. PDPH is caused by poor healing of the puncture site on the dura and arachnoid membrane, which have inadequate blood supply. Cerebrospinal fluid leakage lowers intracranial pressure, causing vasodilation of cranial vessels and resulting in vascular headache. Factors associated with its development include the gauge and type of the puncture needle, as well as repeated attempts at puncture.
Prevention:
- Using fine, non-cutting, conical spinal needles (26G).
- Aligning the bevel of the needle parallel to the longitudinal axis of the spine.
- Avoiding repeated punctures.
Treatment options include:
- Rest in the supine position, abdominal binders, acupuncture, administration of analgesics, and intravenous fluids.
- In severe cases, injection of normal saline, 5% glucose solution, or 15–30 mL dextran 40 into the epidural space.
- If necessary, performing an epidural blood patch with autologous blood.
Urinary Retention
Urinary retention is relatively common and may be caused by delayed recovery of the parasympathetic nerve fibers that innervate the bladder, pain from incisions in lower abdominal or perineal surgeries, or patient discomfort with voiding while lying down. Management includes thermal application, acupuncture, or intramuscular injection of the parasympathomimetic agent carbachol. In some cases, catheterization is necessary.
Neurological Complications After Spinal Anesthesia
Cranial Nerve Palsy
Symptoms usually begin one week after spinal anesthesia and may involve severe headache, photophobia, and dizziness, followed by strabismus and diplopia. This is thought to result from cerebrospinal fluid leakage, which diminishes the cushioning effect of cerebrospinal fluid on the brain. When the patient sits or stands, gravity pulls the brain downward, compressing cranial nerves. The abducens nerve, due to its length, is particularly susceptible to stretching or compression, leading to functional impairment. Symptoms may resolve spontaneously within six months with treatment aimed at correcting low intracranial pressure and administrating vitamin B complex for symptom relief.
Adhesive Arachnoiditis
This condition typically begins with sensory disturbances that may progress to sensory loss and paralysis. Its etiology is unknown, but it is characterized by chronic proliferative inflammatory reactions in the pia mater and arachnoid, resulting in adhesive obliteration of the subarachnoid and epidural spaces, vessel occlusion, and degenerative changes in the spinal cord and nerve roots.
Cauda Equina Syndrome
This syndrome is characterized by localized sensory and motor deficits in the perineal region and distal lower limbs. Milder cases may involve urinary retention, while severe cases can lead to incontinence. If caused by needle injury, recovery may occur within weeks or months. However, chemical injury has a poorer prognosis for recovery.
Purulent Meningitis
This life-threatening condition can arise from direct or indirect sources such as skin infections or sepsis. Prevention is crucial, as complications can be severe and even fatal.
Complications and Management of Epidural Anesthesia
Intraoperative Complications
Total Spinal Anesthesia
Total spinal anesthesia occurs when most or all of the local anesthetic intended for epidural injection unexpectedly enters the subarachnoid space, resulting in the complete blockade of spinal nerves. Patients may develop respiratory difficulty, hypotension, confusion, or loss of consciousness within minutes post-injection, followed by respiratory arrest and cardiac arrest. Immediate oxygen administration with a pressure mask, urgent endotracheal intubation, artificial ventilation, rapid fluid infusion, vasopressors, and intensified monitoring are required in such situations.
Local Anesthetic Toxicity
Toxic reactions to local anesthetics during epidural anesthesia may result from rapid absorption due to the rich venous plexus in the epidural space or accidental catheterization or damage to blood vessels, allowing direct entry of the anesthetic into the bloodstream. Additionally, exceeding the recommended dosage for local anesthetics is a common cause of toxicity.
Hypotension
Hypotension is primarily attributed to blockade of sympathetic nerves, which results in vasodilation of resistance and capacitance vessels. This issue is particularly prominent during upper abdominal surgeries, where extensive thoracic and lumbar sympathetic blockades occur, sometimes involving cardiac sympathetic nerves and causing bradycardia, thus intensifying hypotension. A higher and broader blockade plane or the direct cardiac depressant effects of local anesthetics may further exacerbate hypotension. Management involves blood pressure stabilizing measures, enhanced monitoring, and regulation of the anesthetic plane.
Respiratory Depression
Epidural anesthesia may impair the functioning of the intercostal and diaphragm muscles, leading to reduced respiratory reserve. However, its effect on resting tidal volume is minimal. Respiratory function generally remains normal if the blockade level is below T8, while reserve ventilation significantly declines at levels above T2. Using lower concentrations of anesthetics can mitigate blockade of motor nerves and minimize respiratory depression. For example, lidocaine at 1%–1.3% concentration is suitable for cervical epidural anesthesia, while 1.3%–1.6% concentrations are used for upper thoracic epidural anesthesia, resulting in minimal respiratory compromise despite high blockade planes.
Nausea and Vomiting
This issue is similar to that observed in spinal anesthesia.
Postoperative Complications
Postoperative complications of epidural anesthesia are generally fewer than those associated with spinal anesthesia. A small number of patients may experience low back pain or temporary urinary retention, which are typically mild. However, severe neurological complications, including paraplegia, may occur in rare cases, emphasizing the importance of prevention.
Neurological Injury
Neurological injury can result from direct damage to spinal nerve roots or the spinal cord caused by the puncture needle or rigid epidural catheter, or from the toxic effects of local anesthetics. Symptoms include localized sensory and/or motor deficits corresponding to the affected nerve distribution. If patients report electric shock-like sensations radiating to the extremities during puncture or catheter placement, epidural anesthesia must be abandoned, and symptomatic treatment may allow recovery within weeks or months.
Transient Neurological Syndrome (TNS)
TNS typically occurs within 24 hours after the effects of spinal anesthesia have resolved. Symptoms include unilateral or bilateral radiating pain from the buttocks to the lower limbs or localized pain in the buttocks or legs. Severity varies, but the condition generally resolves within one week. Physical and imaging examinations typically show no neurological abnormalities. Nonsteroidal anti-inflammatory drugs and, if needed, opioids may help manage symptoms.
Epidural Hematoma
The incidence of epidural hematoma is approximately 2%–6%, with the risk of paraplegia due to hematoma formation being 1 in 20,000. If symptoms such as prolonged anesthesia, delayed muscle weakness, or paraplegia appear after an epidural procedure, these may signify hematoma formation compressing the spinal cord. Early diagnosis and timely laminectomy with hematoma evacuation within eight hours of formation significantly improve outcomes, whereas delays beyond 24 hours typically result in poor recovery.
Anterior Spinal Artery Syndrome
The anterior spinal artery, a terminal vessel, supplies the anterior two-thirds of the spinal cord. Prolonged ischemia or inadequate blood supply in this region may cause spinal cord ischemia and even necrosis, manifesting as a series of symptoms collectively referred to as anterior spinal artery syndrome. Patients may report feelings of heaviness or difficulty turning over, usually without sensory deficits. Some patients recover gradually, while others develop paraplegia. Possible causes include pre-existing arterial sclerosis, high concentrations of epinephrine in the anesthetic causing prolonged arterial constriction, or the continuation of severe hypotension during anesthesia.
Epidural Abscess
Improper aseptic technique during the procedure or passage of the puncture needle through infected tissue may lead to infection of the epidural space, resulting in abscess formation. Clinical signs may include radiating pain, muscle weakness, and paraplegia due to spinal cord and nerve root compression, accompanied by symptoms of infection. Adequate administration of effective antibiotics and early laminectomy with abscess drainage are necessary.
Difficulty or Catheter Breakage During Removal
Difficulty in catheter removal or catheter breakage may occur due to rigidity caused by lumbar vertebrae, ligaments, or paraspinal muscles. Restoring the patient's original puncture position typically allows smooth removal. In cases of persistent difficulty, local anesthetic injection around the catheter site or removal under general anesthesia with muscle relaxation may be required. If a catheter fractures without causing infection or neurological symptoms, surgical removal is generally unnecessary, but close monitoring is required. Surgical removal may still be needed in some cases.
Complications and Management of Caudal Anesthesia
The venous plexus within the sacral canal poses a risk of vascular injury during puncture, leading to rapid absorption of local anesthetic and subsequent toxic reactions. Accidental insertion of the needle into the subarachnoid space at depths exceeding the S2 level may cause total spinal anesthesia. Additionally, postoperative urinary retention is relatively common. For patients with sacral canal abnormalities, infections at the puncture site, difficult puncture, or aspiration of blood, alternative anesthesia methods should be considered.