The process of using local anesthetics to temporarily block the conduction of impulses in certain peripheral nerves, thereby inducing anesthesia in the region innervated by these nerves, is known as local anesthesia. During local anesthesia, patients remain conscious, making it suitable for relatively superficial or localized surgeries. However, it may also interfere with the function of important organs. A thorough understanding of local anatomy, the pharmacological properties of local anesthetics, and standardized operational techniques is required when administering local anesthesia.
Local Anesthetic Agents
Chemical Structure and Classification
The chemical structure of commonly used local anesthetic agents mainly consists of three parts: an aromatic ring, an amine group, and an intermediate chain. The intermediate chain can either be an ester or an amide chain. Based on the intermediate chain, local anesthetics are classified into two categories: ester-type anesthetics (e.g., procaine and tetracaine) and amide-type anesthetics (e.g., lidocaine, bupivacaine, and ropivacaine).
Physicochemical Properties and Anesthetic Performance
The physicochemical properties of local anesthetics determine their efficacy and duration of action. Important parameters include dissociation constant (pKa), lipid solubility, and plasma protein binding rate.
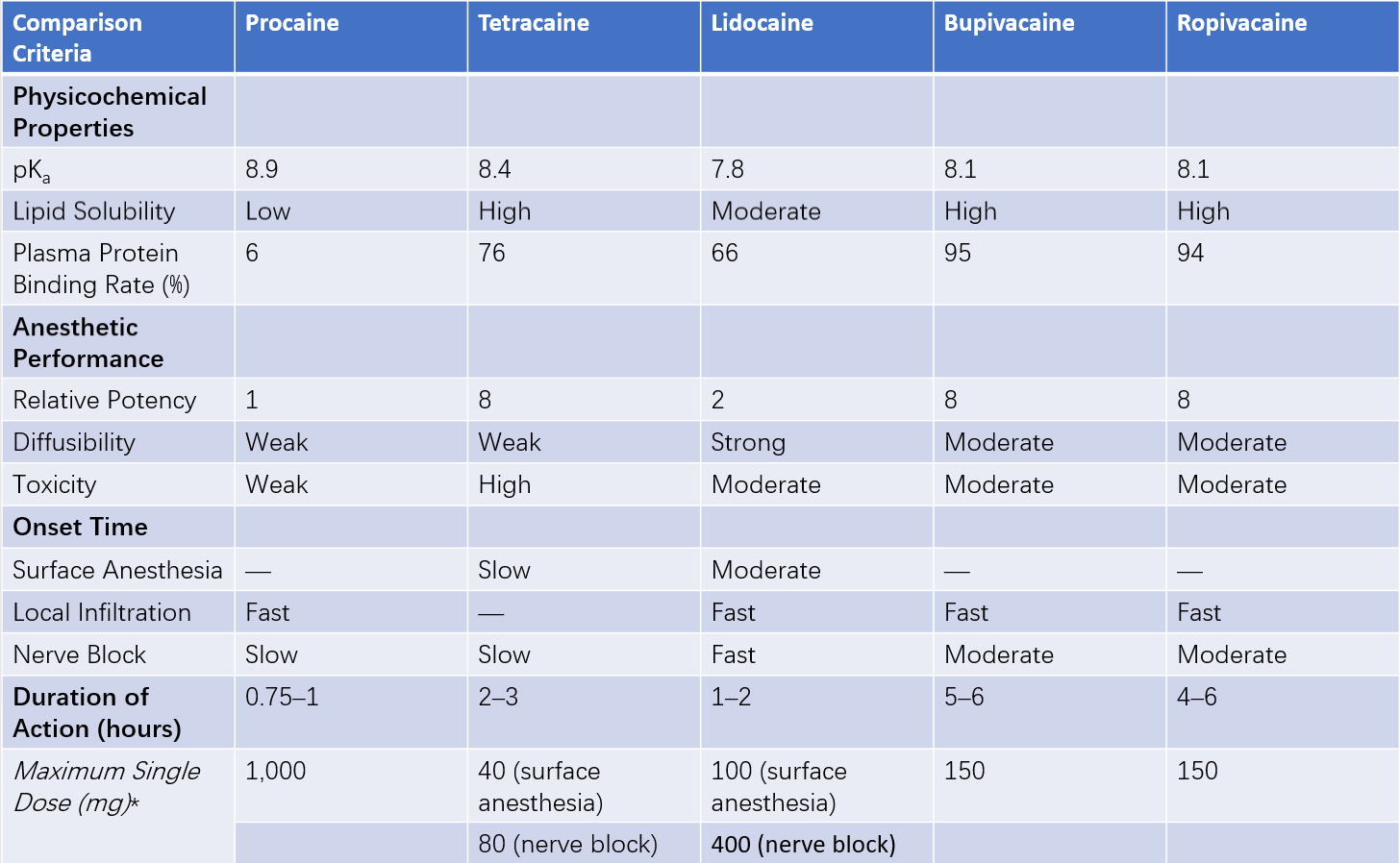
Table 1 Comparison of commonly used local anesthetics
*Note: Maximum doses are for adults and should be adjusted based on the patient’s condition and the specific site of administration.
Dissociation Constant (pKa)
The pKa of local anesthetics is greater than the pH of body fluids. A higher pKa leads to a greater proportion of ionized drug molecules, resulting in reduced diffusion ability and slower penetration through the nerve sheath, thereby increasing the onset time.
Lipid Solubility
Higher lipid solubility corresponds to greater anesthetic potency. Bupivacaine and tetracaine exhibit the highest lipid solubility and anesthetic potency, followed by lidocaine, with procaine being the least potent. Ropivacaine has slightly lower lipid solubility than bupivacaine.
Protein Binding Rate
After injection, local anesthetics exist in both free and protein-bound states. The free form exerts anesthetic effects, while the protein-bound form becomes temporarily pharmacologically inactive. A higher plasma protein binding rate corresponds to a longer duration of action.
Absorption, Distribution, Biotransformation, and Elimination
Absorption
Local anesthetics are absorbed from the site of action into the bloodstream, with the amount and rate of absorption determining the plasma drug concentration. Factors influencing absorption include:
- Drug dosage: Each local anesthetic has a maximum recommended single dose to prevent toxicity from excessive peak plasma concentrations (Cmax).
- Injection site: Absorption varies based on local blood supply. For instance, absorption is faster in intercostal nerve blocks and approaches intravenous injection speeds in alveolar tissues.
- Drug properties: Procaine and tetracaine can cause significant vasodilation at the injection site, accelerating drug absorption.
- Vasoconstrictors: Adding appropriate amounts of vasoconstrictors like epinephrine to local anesthetic solutions reduces absorption, increases duration of action, and decreases toxic reactions. However, bupivacaine and ropivacaine, which bind readily to proteins, have slower absorption rates and do not require additional epinephrine.
Distribution
Once absorbed into the bloodstream, local anesthetics are first distributed to the lungs, where some of the drug is sequestered, buffering its rapid entry into systemic circulation. The drugs are then distributed to highly perfused organs before slowly diffusing into less perfused tissues like muscles, fat, and skin. Due to their high protein binding rates, bupivacaine and ropivacaine have limited ability to cross the placental barrier.
Biotransformation and Elimination
After entering systemic circulation, most local anesthetics are excreted by the kidneys as metabolites, with a small amount excreted in their unchanged form. Amide-based local anesthetics are metabolized in the liver by mitochondrial enzymes; thus, their dosage should be adjusted in patients with impaired liver function. Ester-based local anesthetics are hydrolyzed by plasma cholinesterase. Patients with congenital abnormalities in cholinesterase activity, as well as those with liver cirrhosis, severe anemia, cachexia, or late-stage pregnancy (conditions associated with reduced cholinesterase levels), also require dosage adjustments.
Commonly Used Local Anesthetic Agents
Procaine
Procaine is a short-acting local anesthetic with weak potency and poor mucosal penetration. It is suitable for local infiltration anesthesia but not for surface or epidural anesthesia. Its metabolite, para-aminobenzoic acid, can weaken the effects of sulfonamide drugs, which should be considered during use.
Tetracaine
Tetracaine is a long-acting, potent local anesthetic with strong mucosal penetration. It is appropriate for surface anesthesia, nerve blocks, and spinal anesthesia, but not for local infiltration anesthesia.
Lidocaine
Lidocaine is a medium-potency and medium-duration local anesthetic with excellent tissue diffusion and mucosal penetration. It is suitable for various local anesthesia techniques, with specific concentrations depending on the method. It is most suitable for nerve block and epidural anesthesia. The maximum single dose for adults is 100 mg for surface anesthesia and 400 mg for local infiltration and nerve block. Repeated administration can lead to rapid tolerance.
Bupivacaine
Bupivacaine is a potent, long-acting local anesthetic commonly used for nerve block and spinal anesthesia at concentrations of 0.25%–0.75%. For labor analgesia, concentrations of 0.125%–0.25% are frequently used. Levobupivacaine, a stereoisomer of bupivacaine, has comparable effects but lower cardiac toxicity.
Ropivacaine
The efficacy and pharmacokinetics of ropivacaine are similar to bupivacaine, but ropivacaine demonstrates lower cardiac toxicity. Concentrations of 0.25%–0.75% are typically used for epidural anesthesia, while higher concentrations (0.75%–1%) can effectively block motor nerves. The maximum single dose for adults is 150 mg. Due to its high plasma protein binding rate and selective sensory nerve blockade at low concentrations, ropivacaine is particularly suitable for epidural analgesia, such as postoperative pain relief and labor analgesia.
Methods of Local Anesthesia
Surface Anesthesia
The application of a highly penetrative local anesthetic to mucosal surfaces in order to block nerve terminals located beneath the mucosa and induce localized anesthesia is referred to as surface anesthesia. It is commonly used for superficial surgeries, treatments, or endoscopic examinations involving the eyes, nose, pharynx, trachea, urethra, and skin. Methods include drop instillation for the eyes, application for the nose, spraying for the pharynx and trachea, infusion for the urethra, and topical application for the skin. Commonly used drugs include 1%–2% tetracaine or 2%–4% lidocaine. For the delicate conjunctival and corneal tissues, 0.5%–1% tetracaine eye drops are used, while compound local anesthetic creams are applied to the skin.
Local Infiltration Anesthesia
The injection of a local anesthetic into tissues at the surgical site to block nerve terminals and induce anesthesia is termed local infiltration anesthesia. It commonly involves the use of 0.5% procaine or 0.25%–0.5% lidocaine.
The basic method involves inserting the needle with the bevel downwards into the skin at one end of the surgical incision, injecting the anesthetic to form a wheal. A subsequent injection is made at the edge of this wheal, repeating the process to form a continuous ridge of wheals along the incision line. The principle of step-by-step infiltration is followed, with the local anesthetic injected from the wheal into the subcutaneous tissue, allowing the skin and subcutaneous layers to be incised. In cases where surgery involves deeper tissues, the anesthetic may also be injected intramuscularly, beneath the muscle fascia, or into the peritoneum.
For procedures such as mass excision (e.g., benign breast tumors or scalp surgeries), an alternative method can be employed to avoid directly injecting anesthetic into the tumor tissue and to reduce interference with the anatomical structure of the surgical site. This involves administering the anesthetic circumferentially around the surgical area, including its base, to block the nerves innervating the site. This method is also known as regional blockade.
Precautions include:
- Ensuring the injected anesthetic forms a sufficient volume and tension within the tissues to maximize contact with nerve terminals and enhance the anesthetic effect.
- Reducing the anesthetic concentration to minimize the risk of exceeding the maximum allowable dosage.
- Performing aspiration prior to each injection to avoid intravascular administration.
- Avoiding injection into organs or brain tissues that lack pain sensitivity.
- Using solutions containing epinephrine at a concentration of 1:200,000–1:400,000 (2.5–5 μg/mL) to slow the absorption of the anesthetic, extend its duration of action, and reduce toxicity.
Nerve Block
The injection of a local anesthetic around nerves, plexuses, or ganglia to block impulse conduction and induce anesthesia in the area of innervation is referred to as a nerve block. Common blocks include those of the infraorbital nerve, cervical (brachial) plexus, intercostal nerves, lumbar plexus, sciatic nerve, femoral nerve, and digital nerves. Clinically, stellate ganglion and lumbar sympathetic ganglion blocks are also frequently performed for diagnostic and therapeutic purposes.
Cervical Plexus Block:
The cervical plexus (C1–C4 spinal nerves) is divided into the deep and superficial plexuses. The deep plexus resides between the scalene muscles at the same level as the brachial plexus and is covered by the prevertebral fascia. The superficial plexus emerges from beneath the fascia along the posterior border of the sternocleidomastoid muscle, providing multiple branches that supply the skin and superficial structures.
Deep Plexus Block
Two common techniques are used:
Anterior Cervical Block Method
This often involves blocking at the C4· transverse process. The patient lies supine with the head turned to the opposite side. A line is drawn connecting the mastoid process to the C6 transverse process, and the puncture site lies along this line near the intersection of the C4 transverse process with the posterior border of the sternocleidomastoid muscle and external jugular vein. The transverse process is often palpated by applying pressure. After ensuring no blood or cerebrospinal fluid is aspirated, 10 mL of local anesthetic is injected at a depth of 2–3 cm, where the transverse process is encountered.
Intermuscular Groove Method
This involves the route used for brachial plexus blocks, with the puncture site at the apex of the interscalene groove. After passing through the prevertebral fascia and ensuring no aberrant sensations are elicited, 10 mL of local anesthetic is injected. Compression is applied below the groove to prevent the anesthetic from descending to the brachial plexus.
Superficial Plexus Block
The procedure involves the same positioning as above. A vertical needle insertion is made at the midpoint of the posterior border of the sternocleidomastoid muscle, where 6–8 mL of 1% lidocaine is injected subcutaneously. Alternatively, 3–4 mL is injected at this point, and an additional 2–3 mL is administered along the posterior border of the sternocleidomastoid muscle toward the head and tail ends.
Indications and Complications
Cervical plexus blocks are suitable for neck surgeries, such as thyroid operations and tracheostomies. Complications are rare with superficial plexus blocks but more common with deep plexus blocks, including:
- Toxic reactions to local anesthetics.
- Accidental injection of anesthetic into the subarachnoid space or epidural space.
- Horner syndrome.
- Recurrent laryngeal nerve paralysis.
- Phrenic nerve paralysis (bilateral deep plexus blocks are contraindicated for this reason).
Brachial Plexus Block
The brachial plexus is primarily formed by the anterior rami of C5–C8 and T1 spinal nerves (C and T stand for cervical and thoracic, respectively) and is responsible for the sensory and motor innervation of the upper limb. These nerves emerge from the intervertebral foramina and merge between the anterior and middle scalene muscles within the interscalene groove to form the brachial plexus. The plexus crosses over the first rib above the clavicle before entering the axilla, where it branches into the main terminal nerves: the median nerve, radial nerve, ulnar nerve, and musculocutaneous nerve. Within the interscalene groove, the brachial plexus is enclosed by a sheath formed by the prevertebral and scalene fascia, which extends below the clavicle to form the subclavian arterial sheath and into the axilla as the axillary sheath. Approaches to brachial plexus block include the interscalene, supraclavicular, and axillary routes.
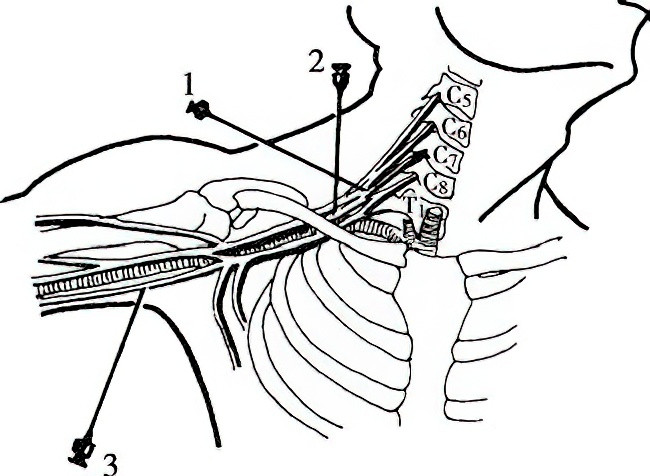
Figure 1 Brachial plexus block
1, Interscalene Approach
2, Supraclavicular Approach
3, Axillary Approach
Interscalene Approach
The patient lies supine with the arm positioned close to the body to allow the shoulder to drop. The head is slightly turned to the opposite side, and the patient raises the head slightly to reveal the clavicular head of the sternocleidomastoid muscle. Using an index finger, the posterior edge of this muscle is palpated and slid outward to locate the interscalene groove—a triangular depression between the anterior and middle scalene muscles, narrower above and wider below. At the level of the cricoid cartilage, the intersection of the groove and the horizontal neck line is marked as the needle insertion point. The needle is inserted perpendicular to the skin and, piercing the prevertebral fascia, a loss of resistance may be felt. The needle is then angled slightly inward and caudally. When the brachial plexus is contacted, the patient may report paresthesia. After confirming the absence of blood or cerebrospinal fluid during aspiration, 15 mL of 1.3% lidocaine or 0.5% ropivacaine is injected.
Supraclavicular Approach
The patient’s position is the same as in the interscalene approach, with a thin pillow placed under the shoulder of the side to be blocked. The needle insertion point is located 1–1.5 cm above the midpoint of the clavicle. The needle is advanced posteriorly, medially, and caudally. When the patient reports paresthesia radiating to the fingers, wrist, or forearm, the needle position is fixed, and after confirming the absence of blood or air during aspiration, the local anesthetic is injected. If paresthesia is absent, a depth of 1–2 cm may allow the needle to contact the first rib. Sliding the needle along the rib until paresthesia occurs allows for anesthetic injection. A fanning technique along the surface of the rib may also be employed for the block.
Axillary Approach
The patient lies supine with the affected arm abducted to 90° and the forearm flexed at 90°, mimicking a saluting posture. The operator palpates the axillary artery at the junction of the edge of the pectoralis major muscle and the medial aspect of the arm. The needle is inserted perpendicular to the skin at either the radial or ulnar side of the artery. Piercing the axillary sheath will result in a distinct loss of resistance. After fixing the needle position, the operator releases the skin and confirms the absence of blood during aspiration. Local anesthetic (20–25 mL) is then injected. Compression of the distal injection site encourages the anesthetic to diffuse proximally within the axillary sheath, facilitating the block of the musculocutaneous nerve.
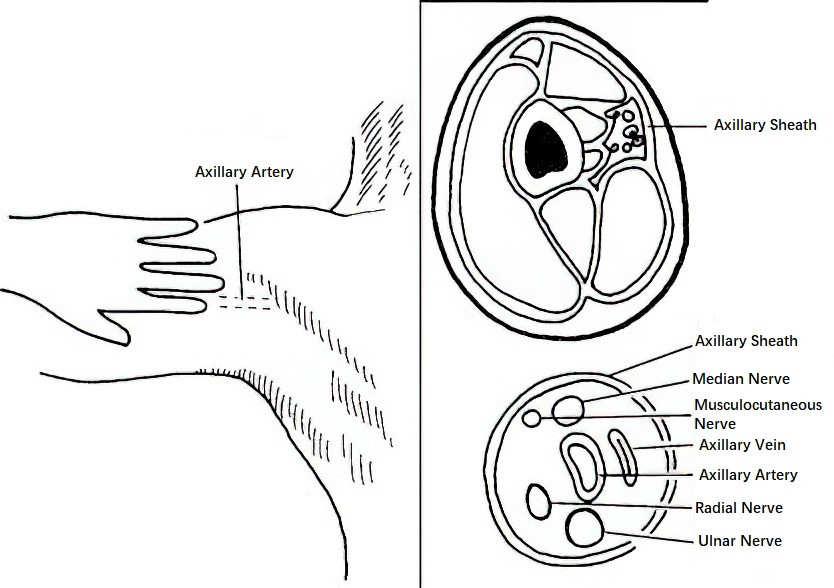
Figure 2 Axillary approach for brachial plexus block
Indications and Complications
Brachial plexus block is suitable for upper limb surgeries. The interscalene approach is used for shoulder surgeries; the supraclavicular approach for surgeries of the elbow, forearm, and hand; and the axillary approach is more specifically for forearm and hand surgeries. Potential complications include toxic reactions to local anesthetics. The interscalene and supraclavicular approaches may result in phrenic nerve paralysis, recurrent laryngeal nerve paralysis, or Horner syndrome. Supraclavicular blocks are more likely to cause pneumothorax, whereas the interscalene approach has a higher risk of high-level epidural anesthesia or inadvertent subarachnoid injection, potentially leading to total spinal anesthesia.
Intercostal Nerve Block
The anterior rami of T1–12 spinal nerves form intercostal nerves, which run in a circumferential path around the trunk. At the costal angle, the nerves lie within the costal groove adjacent to the artery before extending forward. Beyond the anterior axillary line, the nerves, along with blood vessels, are positioned between the internal and external intercostal muscles, where lateral cutaneous branches emerge at this location. Intercostal nerves provide motor innervation to intercostal and abdominal wall muscles and sensory innervation to corresponding skin regions.
Block Method
The patient is positioned laterally or prone, with a pillow under the abdomen and the upper limb abducted. At 6–8 cm lateral to the posterior midline, the rib corresponding to the targeted nerve is identified by palpation, and the puncture site is marked at the corresponding angle of the rib. After skin disinfection and forming a wheal, one hand gently pushes the skin upward. A needle is inserted vertically to touch the lower edge of the rib. Once in contact with the rib, the skin is released; the needle, with the skin moving downwards, slides beneath the rib and is advanced inward by 0.2–0.3 cm. After confirming there is no blood or gas during aspiration, 3–5 mL of local anesthetic is injected. This block is effective for anesthesia and analgesia in corresponding surgical regions. However, risks include pneumothorax and toxic reactions to the local anesthetic.
Digital Nerve Block
This technique is used for surgeries involving the fingers or toes. Each digit’s innervation includes four digital nerves, with contributions from both palmar and dorsal digital nerves.
Proximal Phalangeal Block
The needle is inserted dorsally at the base of the finger and advanced subcutaneously toward the palmar side, sliding past the phalanx. When the needle tip is palpable on the palmar side, it is withdrawn slightly by 0.2–0.3 cm, and 1 mL of 1% lidocaine is injected. Following retraction of the needle to the subcutaneous layer at the initial insertion site, 0.5 mL is injected, and the other side of the finger is blocked similarly.
Intermetacarpal Block
A needle is introduced from the dorsum of the hand between the metacarpal bones, advancing to the subcutaneous layer on the palmar side. While advancing and retracting the needle, 4–6 mL of 1% lidocaine is injected. Skin temperature and color changes are monitored. The use of epinephrine with local anesthetics is contraindicated for digits, toes, and genitalia to prevent ischemic necrosis due to vascular constriction or compression. Excessive local anesthetic volume is also avoided.
In recent years, the use of ultrasound-guidance for neural and fascial plane blocks has allowed for real-time visualization, replacing the conventional "blind searching" method. This approach reduces the volume of local anesthetic required and minimizes complications, improving both safety and comfort. Furthermore, the development of liposomal bupivacaine, a long-acting anesthetic, has effectively addressed the need for prolonged local anesthesia and postoperative analgesia.
Complications of Local Anesthesia and Their Management
Toxic Reactions
Systemic toxic reactions to local anesthetics may occur when excessive amounts are administered, or when the drug is accidentally introduced into blood vessels or the intrathecal space, leading to plasma concentrations exceeding the safety threshold. Severe cases can be life-threatening.
Common Causes
Common causes include
- The administered dose exceeding the patient’s tolerance.
- Accidental intravascular injection.
- Rapid absorption due to injection into areas with rich blood supply.
- Reduced tolerance caused by patient factors such as general debility.
Clinical Manifestations
Symptoms primarily involve the central nervous system (CNS) and cardiovascular system, with the CNS being more sensitive to local anesthetics.
Mild toxic reactions may present as dizziness, verbosity, drowsiness, chills, anxiety, agitation, and disorientation. Early manifestations are mainly excitatory (e.g., elevated blood pressure and increased heart rate), as inhibitory neurons in the CNS tend to be suppressed more easily than excitatory ones.
As toxicity progresses, tremors of the facial muscles and limbs, loss of consciousness, seizures, or convulsions may occur, leading to respiratory and circulatory failure in severe cases. Although local anesthetics primarily inhibit the nervous system, tremors and convulsions may reflect an imbalance in CNS inhibition caused by the drug.
Further increases in plasma concentration result in widespread CNS depression. Cardiovascular toxicity manifests as blockage of sympathetic or parasympathetic nerve fibers, leading to widespread vasodilation, reduced myocardial contractility, bradycardia, atrioventricular block, decreased cardiac output, hypotension, and in extreme cases, cardiac arrest.
Prevention and Treatment
Prevention
The total drug dose must remain within the recommended limit. Aspiration should be performed prior to injection to confirm that the needle is not in a blood vessel. Dosages should be reduced based on the injection site and individual patient factors. Epinephrine can be added to the drug solution in appropriate amounts, and benzodiazepines can be given as premedication to reduce the risk of seizures.
Treatment
Upon the occurrence of toxic reactions, the administration of local anesthetics should be immediately discontinued, and oxygen should be provided. For mild toxic reactions, intravenous diazepam at 0.1 mg/kg or 3–5 mg of midazolam can be administered to prevent and control seizures. For recurrent convulsions, an additional intravenous dose of 3–5 mg midazolam may be given. If endotracheal intubation is feasible, intravenous succinylcholine at 1–2 mg/kg can be used to control respiration. Hypotension may be managed with norepinephrine combined with fluid resuscitation, while atropine can be administered intravenously to address bradycardia. In the event of cardiac arrest, cardiopulmonary resuscitation (CPR) should be initiated without delay. Lipid emulsion therapy with 20% fat emulsion can also be used to treat local anesthetic toxicity, employing a loading dose of 1.5 mL/kg via intravenous injection over one minute, followed by a maintenance infusion at 0.25 mL/(kg·min). Infusion should continue for 10 minutes after circulatory stability is achieved.
Allergic Reactions
Allergic reactions are more common with ester-type local anesthetics and are rare with amide-type agents. Cross-reactivity may occur among drugs within the same class of local anesthetics. Occasionally, reactions related to the addition of epinephrine may resemble local anesthetic toxicity and lead to misdiagnosis as an allergic reaction.
Clinical Manifestations
Symptoms can arise after administering even a small amount of the local anesthetic, including urticaria, pharyngeal edema, bronchospasm, hypotension, or angioneurotic edema. Severe reactions can pose a threat to the patient's life. A careful inquiry into the patient’s allergy history and close monitoring during anesthesia are crucial.
Treatment
Upon the occurrence of an allergic reaction, the drug administration should be stopped immediately. Airway patency should be maintained, and oxygen should be provided. Circulatory stability must be maintained with appropriate fluid supplementation. In emergency situations, vasopressors may be employed. Corticosteroids and antihistamines can be used as adjunctive treatments, though their prophylactic efficacy is debatable. Skin testing for predicting allergic reactions to local anesthetics has limited reliability, with a false positive rate as high as 40%. Routine skin testing is therefore unnecessary. If a patient has a history of allergy to ester-type local anesthetics, amide-type agents may be considered as alternatives.