General anesthesia refers to a reversible condition induced by anesthetic agents administered via inhalation, intravenous injection, or intramuscular injection. In this state, the patient experiences a loss of consciousness, amnesia, analgesia, muscle relaxation, and autonomic nervous system blockade. The degree of central nervous system suppression caused by anesthetic drugs is directly related to the drug concentration within the patient. The depth of anesthesia can be regulated based on the administered dose. The effects of anesthesia on the nervous system are completely reversible, with the patient’s consciousness, pain perception, and reflexes returning to their preoperative state once the drugs are metabolized or eliminated from the body.
General Anesthetic Agents
In a broad sense, general anesthetic agents refer to the diverse range of drugs used during general anesthesia, including inhalation anesthetics, intravenous anesthetics, muscle relaxants, and narcotic analgesics. In a narrower sense, general anesthetics are agents that act directly on the central nervous system to induce general anesthesia. Depending on their route of administration and mechanism of action, general anesthetics are categorized into inhalation anesthetics and intravenous anesthetics.
Inhalation Anesthetics
Inhalation anesthetics are drugs administered via the respiratory tract to induce general anesthesia. These include potent volatile anesthetics (e.g., sevoflurane, desflurane, isoflurane) and nitrous oxide (N2O, commonly referred to as laughing gas). Inhalation anesthetics enter the alveoli via the respiratory tract and diffuse along a concentration gradient through the alveolar membrane into the bloodstream, subsequently reaching the central nervous system to exert their anesthetic effects. Once the administration of inhalation anesthetics ceases, the drugs diffuse back from the central nervous system into the bloodstream and are finally exhaled through the respiratory tract.
Physicochemical Properties and Pharmacological Effects
During inhalation anesthesia, the depth of anesthesia correlates with the partial pressure of the anesthetic within the brain. When the partial pressures of the anesthetic in the alveoli, blood, and brain tissue reach equilibrium, the concentration of the anesthetic in the alveoli indirectly reflects its concentration in the brain, thereby indicating the depth of anesthesia.
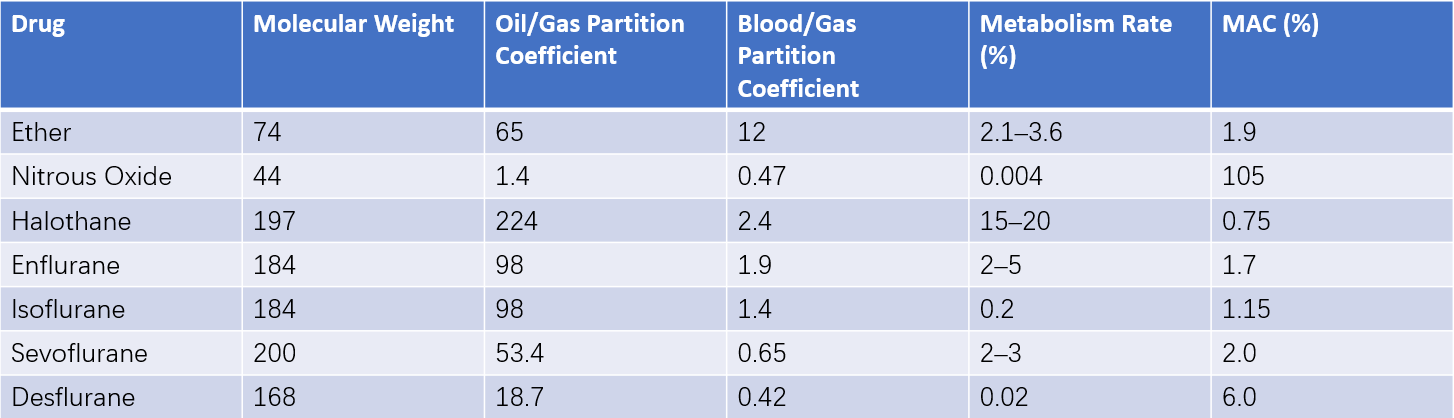
Table 1 Physicochemical properties of inhalational anesthetics
The minimum alveolar concentration (MAC) refers to the alveolar concentration of an inhalation anesthetic, at one atmosphere, which prevents motor responses to surgical incision in 50% of patients. MAC serves as a measure of the potency of different inhalation anesthetics; a lower MAC reflects greater anesthetic potency. The potency of inhalation anesthetics is directly proportional to their oil/gas partition coefficient (a measure of lipid solubility). A higher oil/gas partition coefficient results in stronger anesthetic potency and a lower MAC.
The controllability of inhalation anesthetics depends on their blood/gas partition coefficient (a measure of solubility in the blood). Anesthetic agents with a lower blood/gas partition coefficient reach equilibrium in the alveoli, bloodstream, and brain tissue more quickly, allowing easier control of their concentration in the central nervous system. For example, nitrous oxide, desflurane, and sevoflurane have low blood/gas partition coefficients, enabling rapid induction and recovery.
Metabolism and Toxicity
Most inhalation anesthetics exhibit high lipid solubility and are not readily excreted in unchanged form by the kidneys. The majority are eliminated unchanged through the respiratory tract, while a small fraction is metabolized in the liver and then excreted by the kidneys. Hepatotoxicity and nephrotoxicity are generally low for most inhalation anesthetics. However, the oxidative metabolites of the earliest halogenated anesthetic, methoxyflurane, were associated with fulminant immune-mediated liver damage. Other halogenated agents such as enflurane, isoflurane, and desflurane also carry a potential risk of severe liver damage, though this is extremely rare in clinical practice.
Commonly Used Inhalation Anesthetics
Currently, clinically used inhalation anesthetics include nitrous oxide (N2O), sevoflurane, isoflurane, and desflurane.
Nitrous Oxide
Nitrous oxide is a weak anesthetic and cannot be used alone to maintain anesthesia. It is typically combined with other general anesthetics, with a usual concentration of 50–70%. When used at an inhaled concentration of 50%, nitrous oxide is effective as an analgesic for dental or obstetric procedures. During anesthesia, the inspiratory oxygen concentration (FiO2) must be maintained above 0.3 to prevent hypoxemia. In the recovery phase, the rapid diffusion of nitrous oxide from the bloodstream to the alveoli can temporarily lower the alveolar oxygen partial pressure below the oxygen partial pressure in the air, causing hypoxia, a phenomenon known as diffusion hypoxia. Administering pure oxygen for 5–10 minutes after discontinuing nitrous oxide can mitigate this effect. Nitrous oxide is not advised for patients with conditions involving closed cavities (e.g., middle ear or bowel obstruction) due to the risk of increased pressure within these spaces.
Sevoflurane
Sevoflurane is a potent inhalation anesthetic with a low blood/gas partition coefficient, allowing for excellent control over the depth of anesthesia. It does not irritate the airway or increase secretions and can relax airway smooth muscle, making it suitable for both induction and maintenance of anesthesia. At maintenance concentrations of 1.5–2.5%, it has minimal cardiovascular effects. Sevoflurane enhances the action of non-depolarizing muscle relaxants, reducing the required dose. Recovery from anesthesia is rapid, with an average awakening time of 10 minutes for adults and 8.6 minutes for children.
Desflurane
Desflurane is less potent than sevoflurane but offers strong control over anesthesia depth and exhibits minimal hepatotoxicity and nephrotoxicity. It can be used alone or combined with nitrous oxide to maintain anesthesia. Desflurane also suppresses neuromuscular junctions, reducing the dose of muscle relaxants required during surgery. Due to its limited effects on cardiovascular function, desflurane may offer advantages for cardiac surgery or non-cardiac procedures in patients with heart disease. Its rapid induction and recovery properties make it suitable for outpatient surgeries, with a significantly lower incidence of postoperative nausea and vomiting compared to other inhalation anesthetics. However, desflurane requires special vaporizers and is relatively expensive.
Intravenous Anesthetics
Intravenous anesthetics refer to drugs administered through intravenous injection that act on the central nervous system via the circulatory system to produce general anesthesia. Compared to inhalation anesthetics, intravenous anesthetics have several advantages, including rapid induction, no irritation to the respiratory tract, no environmental pollution, and a lower incidence of postoperative nausea and vomiting. Common intravenous anesthetics include the following:
Ketamine
Ketamine has significant analgesic effects. It can increase cerebral blood flow, intracranial pressure, and cerebral metabolic rate, requiring caution in patients with elevated intracranial pressure. It exhibits sympathomimetic effects, causing an increase in heart rate, blood pressure, and pulmonary artery pressure, making it suitable for induction of anesthesia in patients with shock. Its effects on respiration are minimal, although excessive doses, rapid injection, or combination with other opioid analgesics may cause pronounced respiratory depression and even apnea. The commonly used intravenous induction dose for general anesthesia is 1–2 mg/kg, and the maintenance dose is 15–45 μg/(kg·min). It is also frequently used for pediatric basal anesthesia, with intramuscular administration of 5–10 mg/kg maintaining anesthesia for approximately 30 minutes. Major side effects include transient apnea, increased respiratory secretions, hallucinations, nightmares, psychiatric symptoms, and increased intraocular and intracranial pressures.
Etomidate
Etomidate is a short-acting hypnotic with rapid onset and lacks analgesic effects. It can reduce cerebral blood flow, intracranial pressure, and cerebral metabolic rate. Its impact on heart rate, blood pressure, and cardiac output is minimal, and it does not increase myocardial oxygen consumption. Additionally, it possesses mild coronary vasodilatory effects. Etomidate is primarily used for induction of general anesthesia and is suitable for elderly, frail, or critically ill patients. The typical dose is 0.15–0.3 mg/kg. Side effects include myoclonus following injection, venous irritation leading to local pain at the injection site, postoperative nausea and vomiting, and potential suppression of adrenal cortex function with repeated use or continuous infusion.
Propofol
Propofol provides sedative, hypnotic, and mild analgesic effects. It has a rapid onset, inducing sleep within 30–40 seconds after intravenous injection of 1–2 mg/kg, and patients wake up quickly and fully after discontinuation. Propofol can reduce cerebral blood flow, intracranial pressure, and cerebral metabolic rate. It has a pronounced inhibitory effect on the cardiovascular system, with high doses or rapid injections potentially causing significant reductions in peripheral resistance and cardiac output. Its respiratory depression is dose-dependent. Propofol is metabolized by the liver, and although some accumulation occurs with repeated injections or continuous infusion, it has no significant impact on liver or kidney function. Propofol is widely used as an induction agent for anesthesia and for maintenance during intravenous anesthesia. Side effects include venous irritation causing localized pain at the injection site, respiratory depression necessitating artificial respiratory support as required, and a postoperative nausea and vomiting incidence of approximately 2–5%.
Midazolam
Midazolam has dose-dependent effects that include anxiolysis, sedation, hypnosis, anterograde amnesia, anticonvulsant effects, and central muscle relaxation. It does not cause accumulation with repeated injections. Its effects on the cardiovascular system are minimal, though it may cause mild tachycardia and hypotension. Respiratory depression is dose-dependent. Midazolam decreases intracranial pressure, cerebral blood flow, and oxygen consumption. It is metabolized by the liver and excreted by the kidneys. Clinically, it is commonly used for preoperative sedation, anesthesia induction and maintenance, as an adjunct to local anesthesia, and for sedation in intensive care unit (ICU) patients. Side effects include localized pain at the injection site, thrombotic phlebitis, and anterograde amnesia.
Dexmedetomidine
Dexmedetomidine is a selective α2-adrenergic receptor agonist administered via non-oral routes. It produces dose-dependent sedative, anxiolytic, and analgesic effects and reduces the requirement for opioid medications in combination therapy. It is metabolized by the liver and excreted by the kidneys. Clinically, dexmedetomidine is commonly used for intraoperative sedation, as an adjunct to general anesthesia, and for sedation in mechanically ventilated patients. Side effects include bradycardia, cardiac conduction inhibition, hypotension, and nausea. Excessive sedation may lead to airway obstruction.
Muscle Relaxants
Muscle relaxants, often abbreviated as muscle relaxants for convenience, block neuromuscular transmission and cause skeletal muscle relaxation. These drugs are an important component of general anesthesia. Although muscle relaxants do not themselves produce anesthetic effects, they facilitate skeletal muscle relaxation, improve surgical conditions, and help reduce the required dosage of general anesthetic agents, thereby avoiding the risks associated with deep anesthesia.
Mechanism of Action and Classification
The neuromuscular junction consists of a presynaptic membrane, a postsynaptic membrane, and a synaptic cleft in between. Under physiological conditions, when a nerve impulse reaches the motor nerve terminal, it causes vesicles within the terminal to rupture, releasing acetylcholine into the synaptic cleft. Acetylcholine binds to receptors on the postsynaptic membrane, leading to depolarization of the membrane and contraction of muscle fibers.
Muscle relaxants interfere with normal neuromuscular transmission at the neuromuscular junction. Based on the mode of interference, muscle relaxants are classified into two types: depolarizing muscle relaxants and nondepolarizing muscle relaxants.
Depolarizing Muscle Relaxants
Succinylcholine is a representative drug in this category. Its molecular structure is similar to acetylcholine, allowing it to bind to acetylcholine receptors and cause depolarization of the postsynaptic membrane, resulting in muscle fiber contraction in bundles. However, succinylcholine's affinity for the receptor is stronger, and it is not easily degraded by acetylcholinesterase at the neuromuscular junction. This results in prolonged depolarization of the postsynaptic membrane, preventing repolarization and rendering it unresponsive to acetylcholine released during subsequent nerve impulses, ultimately causing muscle relaxation. Neuromuscular transmission resumes only when the concentration of succinylcholine at the junction decreases and the postsynaptic membrane repolarizes.
Key characteristics of depolarizing muscle relaxants include:
- Binding to acetylcholine receptors on the postsynaptic membrane and causing sustained depolarization.
- Inducing muscle fiber bundle tremors before muscle relaxation, attributed to uncoordinated contraction of muscle fibers.
- Enhancement of the muscle relaxant effect, rather than antagonism, by acetylcholinesterase inhibitors.
Succinylcholine, as the prototypical depolarizing muscle relaxant, has a rapid onset and short duration of action. Muscle fiber tremors appear within 15–20 seconds after intravenous injection, reaching peak muscle relaxation within one minute. Intravenous administration of 1 mg/kg typically causes apnea for 4–5 minutes, with complete muscle strength recovery within 10–12 minutes. Succinylcholine has minimal hemodynamic effects but may cause a transient increase in serum potassium levels. It does not trigger histamine release and therefore does not induce bronchospasm. It is rapidly hydrolyzed by plasma cholinesterase, and its metabolites are excreted in the urine. Clinically, it is primarily used for tracheal intubation during general anesthesia, at a dose of 1–2 mg/kg administered by rapid intravenous injection.
Side effects include potential occurrences of bradycardia and arrhythmias, elevated serum potassium levels during widespread skeletal muscle depolarization, and increased intraocular, intracranial, and intragastric pressures during intense muscle contractions. Postoperative muscle pain is also common.
Nondepolarizing Muscle Relaxants
Tubocurarine is the representative drug in this group. These drugs bind to acetylcholine receptors on the postsynaptic membrane without causing depolarization. When 75–80% or more of acetylcholine receptors on the postsynaptic membrane are occupied by nondepolarizing muscle relaxants, the release of acetylcholine at the nerve terminal is insufficient to bind to the remaining available receptors. This prevents depolarization of the postsynaptic membrane, effectively blocking neuromuscular transmission. The competitive binding of nondepolarizing muscle relaxants and acetylcholine to receptors demonstrates clear dose dependence.
Cholinesterase inhibitors (e.g., neostigmine) slow acetylcholine breakdown, increasing its concentration and allowing it to compete with nondepolarizing muscle relaxants for receptors. Once acetylcholine binding reaches the threshold, postsynaptic membrane depolarization and muscle contraction occur, thus antagonizing the effects of nondepolarizing muscle relaxants.
Key characteristics of nondepolarizing muscle relaxants include:
- Binding to acetylcholine receptors on the postsynaptic membrane without causing depolarization.
- The amount of acetylcholine released from the presynaptic membrane during nerve stimulation remains unchanged but is unable to exert its effects due to receptor blockade.
- Absence of initial muscle fiber bundle contractions before muscle relaxation.
- Reversibility of muscle relaxation effects by cholinesterase inhibitors.
Common nondepolarizing muscle relaxants include vecuronium, rocuronium, and cisatracurium. Vecuronium is potent, with a short duration of action, an onset time of 2–3 minutes, and a clinical action duration of 25–30 minutes. Within clinical dosing ranges, it does not trigger histamine release or vagolytic effects, making it suitable for patients with ischemic heart disease. It is primarily metabolized and excreted by the liver and kidneys, with prolonged action and potential accumulation in patients with significant hepatic or renal impairment.
Rocuronium is the fastest-acting nondepolarizing muscle relaxant currently in clinical use. At a dose of 1.2 mg/kg, tracheal intubation can be performed within 60 seconds. It has a specific reversal agent capable of rapidly antagonizing the neuromuscular blockade caused by rocuronium.
Cisatracurium has an onset time of 2–3 minutes and a clinical duration of 50–60 minutes. Within clinical dosing ranges, it does not induce histamine release and undergoes Hofmann degradation as its primary metabolic pathway, with minimal dependence on liver and kidney function.
Considerations in the Use of Muscle Relaxants
Maintenance of an artificial airway (e.g., tracheal intubation or supraglottic airway device) and implementation of assisted or controlled ventilation are necessary.
Muscle relaxants lack sedative and analgesic properties and should not be used alone but in combination with other general anesthetics.
Succinylcholine may temporarily increase serum potassium, intraocular pressure, and intracranial pressure, and is contraindicated in patients with severe trauma, burns, paraplegia, glaucoma, or elevated intracranial pressure.
Hypothermia can prolong the duration of muscle relaxants, and agents such as inhalation anesthetics, certain antibiotics (e.g., streptomycin, gentamicin, and polymyxins), and magnesium sulfate can enhance the effects of nondepolarizing muscle relaxants.
Nondepolarizing muscle relaxants should be used with caution in patients with neuromuscular junction disorders, such as myasthenia gravis.
Some muscle relaxants may trigger histamine release, requiring careful consideration in asthmatic individuals or those with a history of allergies.
Narcotic Analgesics
Mechanism of Action and Classification
Common narcotic analgesics are opioid drugs that act by binding to opioid receptors in the body. These receptors are primarily located in the pain transmission regions of the brain and spinal cord, as well as areas related to emotional and behavioral regulation. Opioid receptors are generally divided into three subtypes: μ (mu), κ (kappa), and σ (sigma). Stimulation of different receptors results in varying effects.
Common Narcotic Analgesics
Morphine
Morphine is an opioid drug extracted from opium. It acts on the limbic system of the brain to alleviate tension and anxiety and induces a sense of euphoria. Morphine is addictive. It raises the pain threshold and relieves pain. Morphine has significant inhibitory effects on the respiratory center, causing reductions in respiratory frequency in mild cases and decreases in tidal volume or even respiratory arrest in severe cases. It triggers histamine release, which may lead to bronchospasm. Morphine also causes dilation of small arteries and veins, decreases peripheral vascular resistance, and reduces venous return, potentially leading to lower blood pressure, although it does not have a noticeable inhibitory effect on the myocardium. Morphine is primarily used to treat moderate to severe pain, such as excruciating pain from trauma, surgery, or angina pectoris. Due to its effective sedative and analgesic properties, morphine is commonly used for preoperative pain relief, intraoperative anesthesia, and postoperative pain relief.
Pethidine (Meperidine)
Pethidine has analgesic, sedative, and smooth muscle antispasmodic effects. It induces euphoria and is addictive. Pethidine inhibits myocardial contractility, leading to reduced blood pressure and cardiac output. It has mild respiratory depressant effects. Pethidine is most commonly used to treat acute pain and can be combined with drugs such as promethazine or droperidol as an adjuvant for local anesthesia. It is not recommended for children under the age of two.
Fentanyl
Fentanyl is a chemically synthesized, highly potent opioid analgesic with addictive potential. Its analgesic potency is 75–125 times that of morphine, and its effects last for approximately 30 minutes. Fentanyl suppresses respiration but rarely causes hypotension at therapeutic doses used for analgesia or anesthesia. It is commonly utilized for anesthesia induction, intraoperative anesthesia maintenance, and postoperative pain management. It helps to alleviate the cardiovascular responses induced by tracheal intubation. It can also serve as an adjuvant in local anesthesia and is a commonly used anesthetic for cardiovascular surgeries.
Remifentanil
Remifentanil is an ultra-short-acting analgesic. When used alone, it can cause a significant reduction in heart rate. When combined with other general anesthetics, severe hypotension and bradycardia may occur. Respiratory depression caused by remifentanil is dose-dependent, but spontaneous respiration typically recovers within 5–8 minutes after discontinuation. There is a relatively high incidence of muscle rigidity associated with its use. Remifentanil is used for anesthesia induction and intraoperative anesthesia maintenance. It has a strong inhibitory effect on reactions induced by tracheal intubation. Since its analgesic effects disappear quickly after discontinuation, additional analgesic measures, such as administration of medium- to long-acting analgesics or epidural analgesia, should be implemented before stopping its infusion.
Sufentanil
Sufentanil is a derivative of fentanyl, with an analgesic potency 5–10 times higher than that of fentanyl and a duration of action approximately twice as long. Its respiratory depressant effect is similar to that of equipotent doses of fentanyl but is shorter in duration. Sufentanil has less impact on the circulatory system and is therefore more suitable for anesthesia in cardiovascular surgery. It is commonly used for anesthesia induction, intraoperative maintenance, and postoperative analgesia, as well as an adjuvant during local anesthesia procedures.
Implementation of General Anesthesia
Induction of General Anesthesia
Induction of general anesthesia refers to the process wherein a patient gradually loses consciousness and muscle tone after the administration of anesthetic drugs. During this period, the anesthesiologist establishes an artificial airway (e.g., endotracheal intubation) and manages mechanical ventilation. This phase is referred to as the induction period of general anesthesia. Preparations before induction include ensuring the readiness of anesthesia machines, equipment for endotracheal intubation, and suction devices. Intravenous access is secured, gastrointestinal decompression tubes are opened and suctioned, baseline values for blood pressure and heart rate are measured, and electrocardiogram (ECG) and oxygen saturation (SpO2) monitoring are initiated. The methods of induction for general anesthesia are as follows:
Inhalation Induction
A anesthesia mask connected to pure oxygen is placed over the patient's mouth and nose. An anesthetic vaporizer is activated, allowing the patient to inhale the anesthetic agent. Once the patient loses consciousness and enters a state of anesthesia, a muscle relaxant may be administered intravenously, after which endotracheal intubation or laryngeal mask placement is performed.
Intravenous Induction
Intravenous induction begins with the patient inhaling pure oxygen through a face mask for 2–3 minutes. This increases oxygen reserves and flushes out nitrogen from the lungs and tissues. Based on the patient's condition, an appropriate intravenous anesthetic and dosage, such as propofol, etomidate, or midazolam, is selected. The drug is administered slowly through the vein, and the patient's consciousness, circulation, and respiratory changes are closely observed. After the patient loses consciousness, a muscle relaxant is administered. If breathing ceases, the need for assisted ventilation with an anesthesia mask is evaluated based on the patient’s condition. Endotracheal intubation or laryngeal mask placement is performed when muscle relaxation is achieved. Once intubation is successful, the anesthetic machine is connected immediately to commence mechanical ventilation. Compared to inhalation induction, intravenous induction is faster, more comfortable for the patient, and avoids environmental contamination; however, the stages of anesthesia depth are less distinct, and certain intravenous anesthetics may cause significant circulatory effects.
Maintenance of General Anesthesia
Maintenance of general anesthesia refers to the management phase between the completion of induction and the end of surgical anesthesia. During this period, the anesthesiologist administers anesthetic drugs, analgesics, and muscle relaxants either continuously or intermittently to maintain an appropriate depth of anesthesia. Additionally, fluid management and vasoactive drugs are used to regulate vital signs, ensure organ perfusion, and maintain homeostasis. The maintenance of general anesthesia can be categorized into two methods based on the route of anesthetic administration: inhalation anesthesia maintenance and intravenous anesthesia maintenance.
Inhalation Anesthesia Maintenance
This method involves the continuous administration of inhalational anesthetics through the respiratory tract to maintain an adequate depth of anesthesia. Commonly used inhalational anesthetics include nitrous oxide, sevoflurane, isoflurane, and desflurane. Nitrous oxide, due to its weak anesthetic potency, poses a risk of hypoxia at high concentrations, making it unsuitable for use as the sole agent for anesthesia maintenance. Volatile anesthetics such as sevoflurane and isoflurane provide strong anesthetic effects. High concentrations induce loss of consciousness and offer some analgesic and muscle relaxant effects, allowing them to be used alone for maintenance of anesthesia.
However, inhalational anesthetics alone are often inadequate for major surgeries. Clinical practice commonly combines nitrous oxide (N2O), oxygen (O2), and volatile anesthetics to maintain anesthesia, with supplemental intravenous analgesics and muscle relaxants administered as needed. When using nitrous oxide, monitoring of inhaled oxygen concentration and SpO2 is required, ensuring oxygen concentration does not fall below 30%. Volatile anesthetics require vaporizers designed for precise concentration control. Continuous monitoring of the concentrations of inhaled and exhaled anesthetic agents may be performed, if available, to allow for optimal anesthesia depth regulation.
Intravenous Anesthesia Maintenance
This method involves the use of intravenous anesthetics to sustain an adequate depth of anesthesia after induction. Intravenous administration can be carried out through bolus injection, multiple injections, or continuous infusion. The choice of drug and method depends on the characteristics of the surgery, the patient, and the pharmacological properties of different drugs. Intravenous anesthetics commonly have weak analgesic and muscle relaxant effects, necessitating the addition of narcotic analgesics and muscle relaxants during maintenance. This balanced anesthesia technique, referred to as total intravenous anesthesia (TIVA), combines intravenous anesthetics, analgesics, and muscle relaxants to achieve the desired clinical effects. TIVA leverages the advantages of different drugs while minimizing the side effects of individual agents. It offers features such as rapid induction, ease of administration, and the absence of environmental pollution. When the timing and dosage are appropriate, TIVA provides a smooth anesthetic course and facilitates swift recovery.
Combined Anesthesia
Combined anesthesia refers to the use of two or more general anesthetic drugs and/or anesthetic methods to complement each other’s strengths and achieve optimal clinical anesthetic effects. However, selecting appropriate drug types, timing, and dosages during combined anesthesia, based on surgical requirements and pharmacological properties, is critical and challenging. Insufficient anesthesia depth may lead to intraoperative awareness and inadequate analgesia, whereas excessive depth may result in intraoperative hypotension, delayed postoperative recovery, and residual muscle relaxant effects, potentially causing severe complications for the patient.
Familiarity with the pharmacological properties of different drugs is essential for anesthesiologists, who need to monitor intraoperative respiratory and circulatory changes closely and observe stress responses caused by light anesthesia. Intravenous anesthetic drugs may be administered using target-controlled infusion pumps designed based on pharmacokinetic and pharmacodynamic properties of the drugs, allowing for more precise concentration targeting. Advanced indicators such as bispectral index (BIS) monitoring and neuromuscular monitoring, if available, can aid in assessing anesthesia depth and guiding intraoperative drug administration.
Assessment of the Depth of General Anesthesia
In the 1930s, Guedel summarized the various signs and manifestations of ether anesthesia depth into distinct stages. The stages of ether anesthesia depth are based on the degree of suppression of consciousness, nociception, reflexes, muscle tone, respiration, and circulatory function. These stages describe the typical, stepwise inhibition of human physiological systems during general anesthesia. Specifically, anesthesia can be divided into three stages: light anesthesia, surgical anesthesia, and deep anesthesia. Because changes in the depth of ether anesthesia occur relatively slowly, and the clinical characteristics of each stage are distinct, the classification is clear and easy to understand. Although modern anesthetics act more rapidly, and the anesthesia depth becomes less delineated in cases such as combined anesthesia, the key principles from ether anesthesia depth staging still hold reference value for clinical practice in assessing the depth of anesthesia.
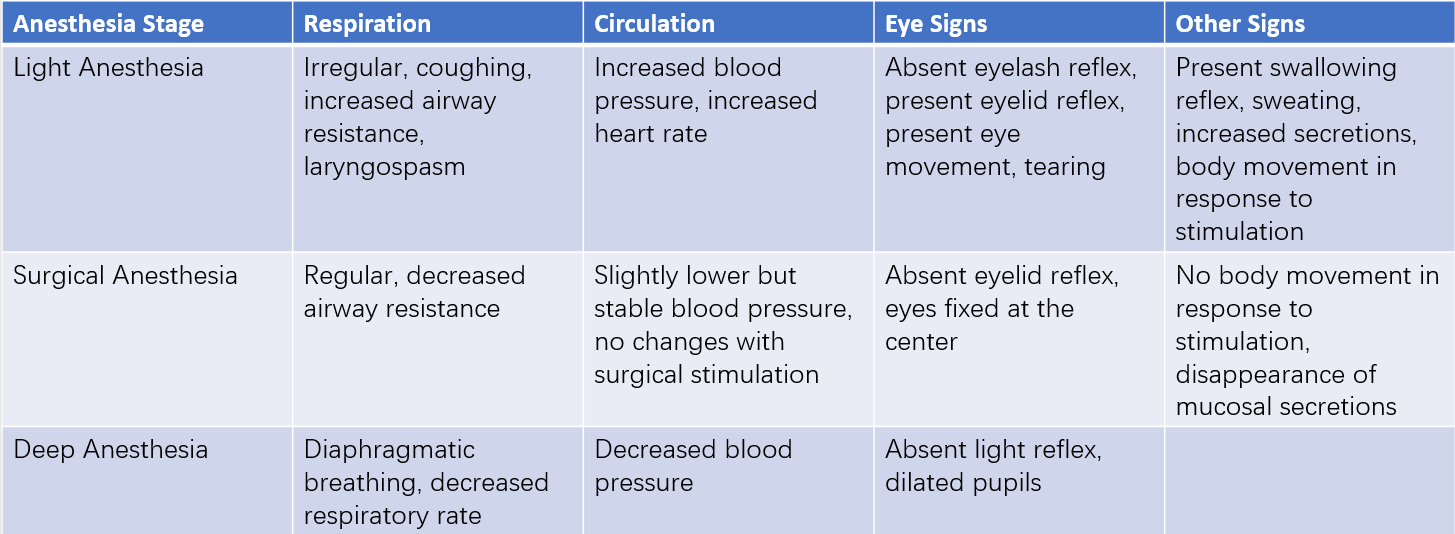
Table 2 Common clinical standards for assessing anesthesia depth
In combined anesthesia, the use of multiple drugs can make determining the depth of general anesthesia more challenging. For instance, the administration of high doses of analgesics and muscle relaxants, even in cases of insufficient anesthesia depth, may result in the absence of pain responses and complete muscle relaxation in the patient. This leads to a situation where the patient remains motionless, and their hemodynamics appear stable during surgery, but the patient’s consciousness is not fully eliminated. The patient is aware of intraoperative events but is unable to express this awareness, a phenomenon known as "intraoperative awareness." Intraoperative awareness during general anesthesia can cause significant psychological harm and even lead to serious adverse consequences.
Assessing anesthesia depth requires a comprehensive evaluation of the effects of combined drugs (including general anesthetics, sedatives, muscle relaxants, and analgesics) on consciousness, sensory perception, motor function, reflexes, and the stability of the internal environment. For example, patients with spontaneous breathing may exhibit increased respiratory rate and respiratory effort in response to surgical stimulation, which indicates light anesthesia. Tearing (lacrimation) is another sign of light anesthesia, while a dry and dull cornea may indicate excessively deep anesthesia. Circulatory stability is also an important factor in assessing anesthesia depth. Severe circulatory suppression often reflects excessively deep anesthesia, whereas tachycardia and elevated blood pressure are more commonly signs of light anesthesia. Maintaining an appropriate depth of anesthesia is a critical yet complex task, requiring the anesthesiologist to closely monitor various indicators and clinical signs. A comprehensive assessment of patient responses is necessary to make reasonable judgments and to adjust anesthesia depth accordingly, based on the intensity of surgical stimuli and the requirements of the procedure.
With ongoing advancements in monitoring technologies, modern clinical practice now includes tools for anesthesia depth monitoring based on electroencephalogram (EEG) waveform analysis, such as bispectral index (BIS) and entropy index monitoring. Various neuromuscular monitoring tools are also available, assisting anesthesiologists in better understanding and managing the patient's depth of anesthesia.
Emergence from General Anesthesia
Emergence from general anesthesia occurs when anesthetics are discontinued at the conclusion of surgery. Inhalational anesthetics are eliminated from the patient’s body through exhalation, while intravenous anesthetics are metabolized and cleared by the liver and kidneys. Residual effects of muscle relaxants are antagonized with reversal agents. The process of removing the endotracheal tube or laryngeal mask airway occurs after the patient regains consciousness, muscle strength, and safe spontaneous breathing ability.
Close monitoring is essential during the emergence process. If the patient exhibits incomplete recovery of respiratory function, significant airway damage, hemodynamic instability, severe hypothermia, or coma, the endotracheal tube or laryngeal mask airway should remain in place postoperatively. The patient should be transferred to the intensive care unit (ICU) for further treatment, and airway devices should only be removed once these conditions improve.
After awakening from general anesthesia, patients are typically transferred to the post-anesthesia care unit (PACU) or monitored further in the operating room until they are fully awake, with stable respiration and circulation. Only when these criteria are met can the patient be transferred out of the monitored environment.
Airway Management
Airway management is a crucial aspect of anesthesia, regardless of the anesthetic technique employed. Its purpose is to ensure a clear airway, maintain the partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2) within safe ranges, prevent lung injury caused by events such as aspiration, and safeguard the patient’s life.
Maintaining Airway Patency
Ensuring airway patency is the foundational requirement of airway management. Common causes of perioperative airway obstruction include posterior displacement of the tongue, oropharyngeal edema and foreign bodies, laryngospasm, and bronchospasm. Depending on the patient’s specific condition, appropriate measures may be taken to ensure the airway remains clear.
Posterior displacement of the tongue is the most common cause of airway obstruction in patients prior to endotracheal intubation during general anesthesia, after extubation, or under sedation in patients not undergoing general anesthesia. Tilting the patient’s head backward or lifting the chin can alleviate obstruction caused by the tongue’s posterior displacement. When necessary, an oropharyngeal or nasopharyngeal airway can be inserted to elevate the base of the tongue and soft tissue of the pharynx, thereby relieving the obstruction. Once the airway obstruction is resolved, ventilation can often be assisted via a face mask.
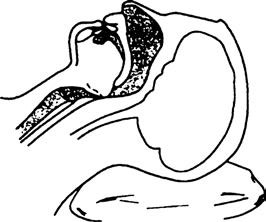
Figure 1 Airway obstruction caused by posterior tongue collapse
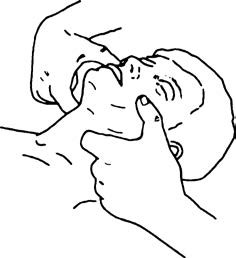
Figure 2 Chin-lift maneuver for maintaining airway patency
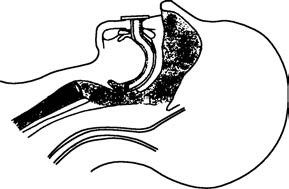
Figure 3 Placement of an oropharyngeal airway
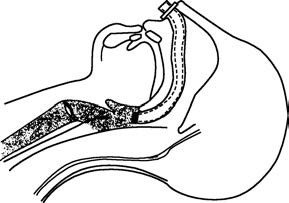
Figure 4 Placement of a nasopharyngeal airway
For patients under general anesthesia or those for whom face mask ventilation is inadequate, endotracheal intubation represents the most commonly used artificial airway management technique. In addition to this, supraglottic airway devices such as the laryngeal mask airway (LMA) and laryngeal tube are also effective approaches for establishing an artificial airway.
Endotracheal Intubation
Endotracheal intubation is a procedure in which a specially designed endotracheal tube is inserted into the patient’s trachea through either the oral or nasal cavity. It is a fundamental skill that anesthesiologists must master and serves as a critical component of clinical anesthesia management. The objectives of endotracheal intubation include:
- Maintaining a patent airway during anesthesia, preventing foreign matter from entering the airway, and facilitating the suctioning of tracheal secretions or blood.
- Enabling mechanical ventilation to prevent hypoxia or carbon dioxide accumulation.
- Allowing the administration of inhalational anesthetics.
Endotracheal intubation is indicated in scenarios where airway patency cannot be assured during the course of general anesthesia (e.g., intracranial surgery, thoracic surgery, or surgeries in the prone position). It is also used in cases where diseases make it difficult to maintain airway patency (e.g., tracheal compression caused by tumors) or where general anesthetics and muscle relaxants significantly suppress respiratory function. Endotracheal intubation is equally vital in the management of critically ill patients, including those requiring mechanical ventilation due to respiratory failure, cardiopulmonary resuscitation, drug poisoning, or severe neonatal asphyxia.
Common methods of intubation include oral and nasal routes:
Oral Intubation
The endotracheal tube is inserted into the trachea via the mouth using a direct laryngoscope to expose the glottis. For difficult visualization of the glottis, additional equipment such as video laryngoscopes, rigid video scopes, or flexible fiber-optic bronchoscopes may assist in the glottic exposure and facilitate intubation. The appropriate insertion depth of the endotracheal tube is measured from the teeth to the tip of the tube, with typical insertion depths being approximately 20–22 cm for adult females and 22–24 cm for adult males. After intubation, confirmation of correct tube placement within the trachea and at the appropriate depth is required based on surgical needs and the patient’s facial anatomy.
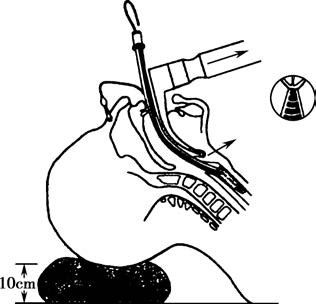
Figure 5 Visualization of the glottis using a laryngoscope
Methods to confirm proper tube placement include:
- Expiratory airflow from the tube when the chest is compressed.
- Symmetric rise and fall of the chest during mechanical ventilation, with bilateral, clear, and equal breath sounds.
- Balloon movement synchronized with spontaneous breathing when the patient resumes self-respiration and the tube is connected to the anesthetic machine.
- Visualization of the endotracheal tube within the trachea using a bronchoscope.
- Presence of a consistent end-tidal carbon dioxide (PETCO2) waveform during ventilation, showing characteristic CO2 patterns, confirming successful intubation.
Nasal Intubation
This method involves inserting the endotracheal tube into the trachea via the nasal cavity in specific scenarios, such as during oral surgery or in patients with limited mouth opening. Nasal intubation can be performed under the assistance of a laryngoscope or bronchoscope or by blind insertion while maintaining the patient’s spontaneous breathing.
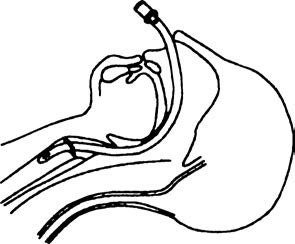
Figure 6 Intubation via the nasal passage into the trachea
Complications of Endotracheal Intubation
Mechanical Injuries
Endotracheal intubation may cause damage or loss of teeth, bleeding from injuries to the oral, pharyngeal, or nasal mucosa, and dislocations of the temporomandibular joint or cricoarytenoid cartilage.
Physiological Responses
Intubation under light anesthesia may provoke intense coughing, breath-holding, laryngospasm, or bronchospasm. Rapid changes in heart rate and blood pressure may increase the risk of myocardial ischemia or cerebrovascular events. Severe vagal reflexes could result in arrhythmias or even cardiac arrest.
Tube-Related Issues
Tubes with an excessively small diameter can increase airway resistance, while overly large or rigid tubes may damage the glottis, leading to acute laryngeal edema, respiratory mucosal injury, or long-term granuloma formation. Soft tubes may deform or kink, causing airway obstruction.
Improper Tube Positioning
Excessively deep insertion may result in the tube entering one main bronchus, leading to inadequate ventilation, hypoxemia, or postoperative atelectasis. Shallow insertion may lead to accidental dislodgment during position changes, causing severe complications. Therefore, verifying tube depth after positional adjustments and routinely auscultating breath sounds in both lungs is necessary.
Laryngeal Mask Airway (LMA)
The laryngeal mask airway is a specialized supraglottic airway management device that has been widely used in clinical anesthesia for over 40 years. It remains one of the most commonly employed supraglottic artificial airway devices in practice. The LMA features an oval-shaped cuff at its distal end, which seals over the epiglottis and glottis, creating a ventilatory space above the vocal cords.
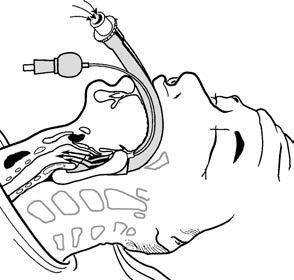
Figure 7 Correct positioning of a laryngeal mask airway (LMA)
After placement, the position of the LMA may be assessed through various means, including auscultation, airway resistance, PETCO₂ waveform monitoring, or nasogastric tube placement in dual-channel LMAs. Properly positioned, LMAs allow for spontaneous breathing or connection to a ventilator for mechanical ventilation.
Advantages of the LMA
The LMA does not require a laryngoscope or muscle relaxants for placement, making the procedure simple, with a high success rate. Beginners can learn to use LMAs effectively after minimal training. These characteristics make LMAs particularly useful in emergency airway management situations outside the operating room. Intubating LMAs can facilitate both artificial ventilation and guidance for endotracheal intubation, especially in cases of both difficult ventilation and challenging intubation.
Since the LMA is a supraglottic device, it does not contact the glottis or trachea, resulting in minimal stimulation to the patient. This reduces the risk of coughing, movement, and hemodynamic fluctuations during insertion or removal. Postoperative sore throat and hoarseness are also less common compared to endotracheal intubation.
However, LMAs cannot fully prevent aspiration, making them unsuitable for patients at high risk of vomiting or reflux (e.g., full stomach, severe gastroesophageal reflux disease, or elevated intra-abdominal pressure). LMA placement requires a mouth opening of at least 2 cm, and it is contraindicated in patients with abnormal pharyngeal anatomy or infection, subglottic airway obstruction, or severe lung disease. Improper LMA positioning or poor seal integrity may lead to gastrointestinal distension during positive-pressure ventilation, increasing the risks of reflux and aspiration.
Complications of General Anesthesia and Their Management
The continuous improvement of anesthesia quality and safety remains the ultimate goal pursued by anesthesiologists. In recent years, advancements in monitoring and management techniques have led to significant reductions in the incidence and mortality of severe perioperative anesthetic complications. However, surgery and anesthesia are inherently complex processes, where patient factors, surgical factors, anesthetic factors, and equipment factors all contribute to the occurrence of perioperative complications. This section focuses on the most common complications of general anesthesia, as well as rare but life-threatening complications.
Regurgitation and Aspiration
During general anesthesia, a patient experiences a loss of consciousness, reduced or absent swallowing and coughing reflexes, and relaxation of the cardia, making regurgitation more likely in patients with significant gastric contents. If the regurgitated materials reach the pharynx, aspiration may occur, potentially leading to asphyxia or aspiration pneumonia. Regurgitation and aspiration typically occur during the induction phase before endotracheal intubation or during emergence after extubation. Patients with a full stomach, lower esophageal sphincter dysfunction, prior esophageal or gastric surgery, impaired gastric emptying, or neuromuscular disorders that result in laryngeal dysfunction are at higher risk of regurgitation and aspiration.
Symptoms often include nausea, vomiting, coughing, increased salivation, frequent swallowing, and spasmodic breathing. Vomiting patients may be turned laterally and placed in a head-down, feet-up position to prevent the entry of vomitus into the airway. Suction devices are used to clear regurgitated material from the oral and nasal cavities. If necessary, endotracheal intubation or bronchoscopy may be employed to remove airway foreign bodies.
The clinical manifestations and prognosis of aspiration depend on factors such as the type of material aspirated, its volume, and its pH. Large-volume aspirations, particularly of solid food, may cause partial or complete airway obstruction, resulting in severe asphyxia, hypoxia, or even cardiac arrest. Aspiration of acidic gastric contents can trigger bronchospasm or asthma-like episodes and lead to aspiration pneumonia, characterized as Mendelson’s syndrome. Symptoms may include cyanosis, dyspnea, shallow and rapid breathing, and tachycardia. Lung auscultation often reveals wheezing or crackles, and chest X-rays may show irregular, blurred patchy opacities in affected lung fields, suggestive of pulmonary edema (commonly seen in the right lower lobe).
Bronchodilators and antibiotics may be administered to prevent lung infections. For confirmed aspiration of gastric contents, bronchoscopy and suctioning are typically performed after intubation, followed by endotracheal instillation of 5–10 ml of normal saline for repeated lavage until the suctioned fluid becomes clear. Corticosteroids may also be administered for 2–3 days. Severe outcomes of regurgitation and aspiration, such as aspiration atelectasis or aspiration pneumonia, are associated with poor prognoses, highlighting the importance of prevention during general anesthesia. For elective surgeries, patients are required to fast and abstain from drinking fluids preoperatively, and surgeries should be postponed for patients with a full stomach. For emergency surgeries involving patients with a full stomach, regional anesthesia or spinal anesthesia can be considered while keeping the patient awake. In cases where general anesthesia is required, preoperative medications to promote gastric emptying and elevate gastric pH may be used. Rapid sequence induction (RSI) is performed during anesthesia induction, which involves preoxygenation followed by sequential administration of fast-acting anesthetic agents (e.g., propofol) and muscle relaxants (e.g., succinylcholine or 1.0–1.5 mg/kg rocuronium). Mask ventilation is avoided while applying cricoid pressure to reduce the risk of regurgitation and aspiration. Endotracheal intubation is performed promptly after muscle relaxation. During emergence, extubation is delayed until the patient is fully awake and protective reflexes of the pharynx and larynx have returned.
Airway Obstruction
Airway obstruction can occur in the upper or lower respiratory tract, with the glottis serving as the dividing point.
Upper Airway Obstruction
Common causes include mechanical blockages such as posterior displacement of the tongue, oral secretions or blood, foreign bodies, laryngeal edema, and laryngospasm. Partial obstruction manifests as difficulty breathing and snoring, while complete obstruction features nasal flaring, chest wall retractions, and no gas exchange despite forceful breathing efforts. Posterior tongue displacement can be alleviated by lifting the chin upward or inserting an oropharyngeal or nasopharyngeal airway. Foreign bodies and secretions in the pharynx must be removed promptly.
Laryngeal edema is more common in infants, young children, and patients who have undergone repeated intubations. It may also occur due to surgical traction or laryngeal irritation. Mild cases typically respond to corticosteroids, but severe cases require endotracheal intubation or tracheotomy to relieve the obstruction.
A common cause of upper airway obstruction is laryngospasm, which often arises from light anesthesia with foreign body irritation of the larynx or during procedures such as urethral dilation, cervical dilation, or anal sphincter manipulation. Laryngospasm presents as inspiratory difficulty, stridor, and cyanosis due to hypoxia. Mild cases usually resolve with oxygen administration under positive pressure delivered via a face mask. Severe cases may require muscle relaxants, controlled ventilation, or cricothyroid membrane puncture with catheter placement for oxygenation. Preventing laryngospasm involves gentle suctioning of the larynx when the patient is either deeply anesthetized or fully awake, as well as ensuring adequate anesthesia depth during painful procedures.
Lower Airway Obstruction
Common causes include bronchospasm, kinking of the endotracheal tube, occlusion of the tube by its beveled tip, and blockages from secretions or aspirated material within the trachea or bronchi. Bronchospasm often occurs in patients with a history of asthma or chronic obstructive pulmonary disease (COPD). These patients often have increased bronchial smooth muscle tone and hyperresponsive airways, and insertion of the endotracheal tube can provoke severe tracheal and bronchial spasms, leading to lower airway obstruction and hindering airflow into the lungs. Auscultation may reveal wheezing or even absent breath sounds in severe cases. Hypoxemia, CO2 retention, and tachycardia may also occur. Maintaining adequate anesthesia depth and oxygenation are critical for mitigating bronchospasm. Ketamine and inhalational anesthetics, which have bronchodilatory properties, are preferred anesthetics for patients with asthma.
In the event of bronchospasm, oxygen concentration is increased, and drugs such as aminophylline (250–500 mg infusion), hydrocortisone (100 mg), or inhaled bronchodilators are used to relax the bronchial smooth muscles and prevent hypoxia.
Hypoventilation
Hypoventilation can occur during anesthesia or after general anesthesia, with its primary manifestation being CO2 retention, potentially accompanied by hypoxemia. During anesthesia, hypoventilation is mainly caused by central and peripheral respiratory depression induced by anesthetic agents, narcotic analgesics, and muscle relaxants, as well as insufficient minute ventilation during assisted or controlled ventilation. Increasing tidal volume and/or respiratory rate is often required. Post-anesthesia hypoventilation mainly results from the residual effects of various anesthetics, particularly narcotic analgesics and muscle relaxants, which lead to central respiratory depression and respiratory muscle dysfunction. Assisted or controlled ventilation may be necessary until the patient's respiratory function fully recovers, with antagonistic drugs being administered if necessary.
Hypoxemia
Hypoxemia is diagnosed when SpO2 is less than 90% and PaO2 is less than 60 mmHg while breathing room air, or when PaO2 is less than 90 mmHg while breathing pure oxygen. Clinical manifestations include rapid breathing, cyanosis, agitation, arrhythmias, and elevated blood pressure. Common causes and their management principles include:
- Faults in the anesthesia machine, insufficient oxygen supply, low inspired oxygen concentration, endotracheal tube insertion into a single bronchus or tube displacement outside the trachea, and airway obstruction, which all may lead to hypoxemia and need to be promptly identified and corrected.
- Diffusion hypoxia, which may occur after inhalational anesthesia with nitrous oxide (N2O), can be improved by continuing oxygen administration for at least 5–10 minutes after discontinuing N2O.
- Atelectasis, which can be managed with suctioning, increased ventilation, and lung recruitment maneuvers.
- Aspiration, which in mild cases responds to oxygen therapy and in severe cases may require mechanical ventilation.
- Pulmonary edema, which may develop in patients with acute left heart failure or increased pulmonary capillary permeability. Increasing oxygen concentration and actively treating the underlying disease are key steps in managing this condition.
Hypotension
Hypotension during anesthesia is defined as a systolic blood pressure drop exceeding 30% of the baseline value or an absolute systolic pressure below 80 mmHg. Common causes include:
- Excessively deep anesthesia, which may reduce blood pressure and narrow pulse pressure, with the effect being more pronounced in patients with preexisting hypovolemia.
- Excessive blood loss during surgery, which may lead to hypovolemic shock.
- Allergic reactions, adrenal insufficiency, and rewarming, which may all lead to reduced vascular tone and hypotension. Treatment includes fluid volume replacement, restoring vascular tone with vasopressors, and addressing the underlying cause.
- Intraoperative visceral traction, which may reflexively lower blood pressure and cause bradycardia. Stimulation should be removed, and atropine may be administered if necessary.
Hypertension
Hypertension during anesthesia is defined as a systolic blood pressure exceeding 160 mmHg or an increase greater than 30% from baseline. This condition may increase blood loss, myocardial oxygen consumption, and the risk of cardiovascular or cerebrovascular complications. Hypertension during surgery is commonly caused by:
- Comorbid conditions such as primary hypertension, pheochromocytoma, hyperthyroidism, primary hyperaldosteronism, or elevated intracranial pressure.
- Surgical or anesthetic factors, including surgical exploration and endotracheal intubation.
- Hypoventilation and resulting CO2 retention.
- Drug-induced hypertension, such as that caused by ketamine.
Management involves addressing the underlying cause and maintaining appropriate anesthetic depth. For refractory hypertension, antihypertensive drugs may be used to stabilize circulation.
Arrhythmia
Arrhythmia can be triggered by inadequate anesthetic depth, excessive surgical stimulation, hypotension, hypertension, CO2 retention, and hypoxemia. Patients with preexisting cardiac diseases, particularly those with a history of arrhythmias, are more prone to developing arrhythmias during anesthesia. When arrhythmia occurs, identifying and eliminating the underlying causes is critical. Maintaining appropriate anesthetic depth, normal circulatory volume, stable hemodynamics, and myocardial oxygen supply-demand balance is important for management. Sinus tachycardia accompanied by hypertension is often indicative of insufficient anesthetic depth and may improve with a deeper level of anesthesia. Hypovolemia, anemia, and hypoxia are common causes of sinus tachycardia and should be addressed appropriately. Visceral traction during surgery (e.g., gallbladder surgery leading to the gallbladder-cardiac reflex) or the occurrence of oculocardiac reflexes can lead to vagal reflex-mediated bradycardia, which in severe cases may result in cardiac arrest. Stopping the surgical operation and administering intravenous atropine, if necessary, can help manage such situations. For premature contractions, the nature of the arrhythmia and its hemodynamic impact should be assessed. Atrial premature contractions typically do not require special treatment if they have no significant hemodynamic effects. Ventricular premature contractions caused by light anesthesia or CO2 retention often resolve after deepening anesthesia or eliminating CO2. However, frequent, multiform, or R-on-T ventricular premature contractions suggest insufficient myocardial perfusion, which requires prompt treatment.
Postoperative Nausea and Vomiting (PONV)
Postoperative nausea and vomiting refer to nausea and/or vomiting occurring within 24 hours after surgery and are among the most common complications following general anesthesia. While most patients experience mild symptoms, they can result in significant discomfort. Severe symptoms in a subset of patients may lead to serious complications, including aspiration pneumonia, dehydration, wound dehiscence, and esophageal rupture.
The risk factors for PONV are varied. The most common risk factors in adults include: female gender, a history of motion sickness or PONV, non-smoking status, and the use of opioid medications. The likelihood of PONV increases with the number of risk factors present. For patients with two or more risk factors, prophylactic administration of antiemetic drugs is recommended. Common antiemetics include 5-HT3 receptor antagonists, glucocorticoids, droperidol, and metoclopramide. In addition, anesthesia strategies for high-risk patients can include avoiding inhalational anesthetics, using a multimodal analgesia approach with nonsteroidal anti-inflammatory drugs, and incorporating nerve blocks to reduce opioid consumption.
Malignant Hyperthermia
Malignant hyperthermia is a condition characterized by an abnormally high skeletal muscle metabolic rate triggered in susceptible individuals with pathogenic genetic mutations during exposure to volatile inhalational anesthetics and/or depolarizing muscle relaxants during general anesthesia. Typical clinical manifestations include sustained muscle spasms, a rapid rise in PaCO2, a sudden and steep increase in body temperature (up to 1°C every 5 minutes), which can exceed 42°C, and severe metabolic acidosis. The most common drugs that precipitate malignant hyperthermia are succinylcholine and halothane. The condition develops suddenly and progresses rapidly. Without timely and effective diagnosis and treatment, the mortality rate is very high. Dantrolene is the specific therapeutic agent for treating malignant hyperthermia.