Septic shock is a common and challenging form of shock encountered in surgical settings. It is characterized by an imbalance in the host-microorganism response and often occurs secondary to infections, predominantly caused by Gram-negative bacteria, such as acute peritonitis, biliary tract infections, strangulated intestinal obstruction, and urinary tract infections. This condition is also referred to as endotoxin shock. Gram-negative bacterial endotoxins bind to components within the body, such as complement proteins, antibodies, or other factors, stimulating the sympathetic nervous system and leading to vascular spasm, endothelial injury, and the release of inflammatory mediators like histamines, kinins, prostaglandins, and lysosomal enzymes. These processes trigger systemic inflammatory response syndrome (SIRS), which ultimately results in microcirculation dysfunction, metabolic disturbances, and organ dysfunction.
The diagnostic criteria for SIRS include:
- Body temperature >38°C or <36°C.
- Heart rate >90 beats per minute.
Rapid breathing (>20 breaths per minute) or hyperventilation with PaCO2 <4.3 kPa (32.29 mmHg).
- Leukocyte count >12 × 109/L or <4 × 109/L, or immature neutrophils >10%.
Septic shock is diagnosed when the following are present simultaneously:
- SIRS.
- Evidence of bacterial infection (positive bacterial culture and/or clinical signs of infection).
- Manifestations of shock.
The hemodynamic patterns of septic shock can be categorized into hyperdynamic and hypodynamic types. The hyperdynamic subtype (high-output, low-resistance) involves peripheral vasodilation, reduced vascular resistance, and normal or increased cardiac output. This is associated with abnormal blood flow distribution, increased arteriovenous shunting, impaired cellular metabolism, and insufficient energy production. Patients in this phase may present with warm, dry skin, often referred to as "warm shock." This condition may occur during the early stages of shock caused by certain Gram-positive bacterial infections.
The hypodynamic subtype (low-output, high-resistance) involves peripheral vasoconstriction, microcirculatory stagnation, and significant capillary leakage, leading to reduced blood volume and cardiac output. Patients in this phase exhibit cold and clammy skin, commonly referred to as "cold shock," which is more frequently seen in Gram-negative bacterial infections. Advanced stages of septic shock caused by Gram-positive bacteria often progress to this "cold shock" phase.
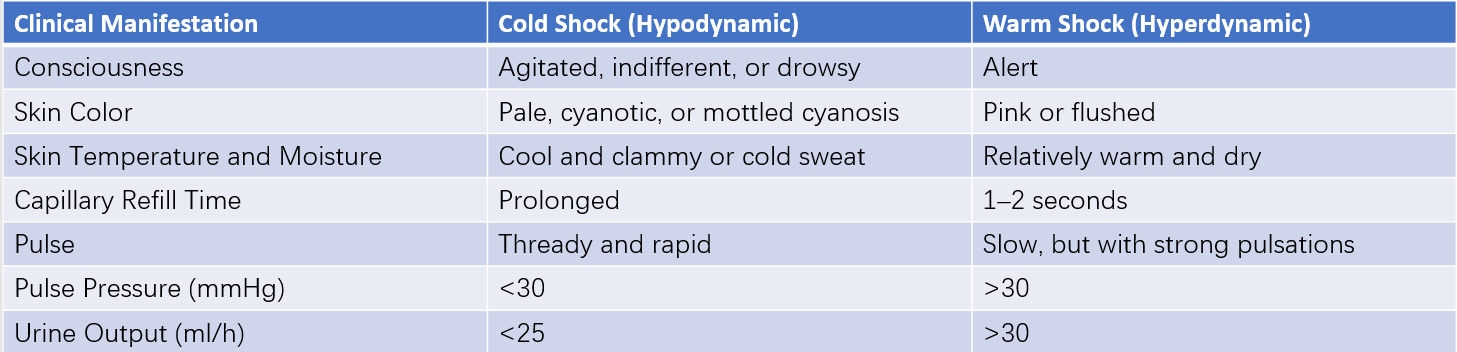
Table 1 Clinical features of septic shock
Treatment
The pathophysiological changes in septic shock are complex, and treatment is challenging. Severe septic shock has a mortality rate ranging from 30% to 50%. For septic shock caused by surgical conditions, treatment primarily involves addressing the underlying cause, often requiring effective surgical drainage (via surgery or percutaneous intervention). Management priorities shift according to the phase: shock and infection are addressed concurrently when shock persists, while infection becomes the primary focus after shock has been stabilized.
The first evidence-based Surviving Sepsis Campaign (SSC) guidelines introduced in 2004 emphasized the concept of a "sepsis bundle" for septic shock and sepsis management. This approach prioritizes early administration of effective antibiotics, rapid correction of tissue hypoxia, and dynamic assessments. The revised 2018 guidelines introduced the concept of the "hour-1 bundle," which remains the current recommendation.

Table 2 Hour-1 bundle recommendations (2018 edition)
Fluid Resuscitation
Treatment of shock initially focuses on the infusion of balanced salt solutions, complemented with appropriate colloid solutions, plasma, or whole blood to restore adequate circulating volume. Central venous pressure (CVP) monitoring is typically performed to maintain normal CVP values. Intermittent red blood cell transfusions may be required to correct anemia, ensuring optimal cardiac filling pressure, arterial oxygen content, and blood viscosity. Since myocardial and renal function is often impaired in patients with septic shock, fluid volume and infusion rates are adjusted based on CVP to avoid adverse consequences of fluid overload.
Infection Control
Major components of infection control include the use of antimicrobial agents and addressing the primary infectious source. For patients without a confirmed pathogen, empirical treatment with broad-spectrum antibiotics may be initiated. When the causative organism is clearly identified, the choice of antibiotics is guided by antimicrobial susceptibility testing. Increasing bacterial resistance necessitates careful antibiotic selection in alignment with specific clinical circumstances. Reliance solely on antibiotics is insufficient, and timely management of the primary infection source is essential.
Correction of Acid-Base Imbalance
Severe acidosis, frequently observed early in septic shock, warrants prompt correction. Alongside fluid resuscitation, 5% sodium bicarbonate solution (200 mL) is administered through an alternate intravenous route, with additional infusions guided by arterial blood gas analysis.
Use of Cardiovascular Agents
If septic shock persists after fluid resuscitation and correction of acidosis, vasodilators may be administered. A combination of medications can be employed, including α-receptor agonists with mild β-receptor stimulation effects (e.g., dopamine) or α-receptor antagonists combined with β-receptor agonists to counteract excessive vasoconstriction while preserving β-receptor effects without excessively increasing heart rate. Examples include anisodamine, dopamine, norepinephrine, or a combination of norepinephrine and phentolamine. Inotropic agents (e.g., dobutamine) may also be administered to improve cardiac function when required.
Glucocorticoid Therapy
Glucocorticoids inhibit the release of inflammatory mediators and stabilize lysosomal membranes, thereby alleviating SIRS. Early administration of high doses (limited to within 48 hours) may be beneficial. However, prolonged use poses risks of severe complications, such as acute gastric mucosal injury and immunosuppression.
Additional Treatments
Other supportive interventions include nutritional support, management of disseminated intravascular coagulation (DIC), and addressing organ dysfunction.