Shock is a pathophysiological process characterized by decreased effective circulating blood volume, insufficient tissue perfusion, disordered cellular metabolism, and impaired cellular function. Insufficient tissue perfusion results in disturbances in oxygen delivery, transport, and utilization by tissues, leading to metabolic abnormalities, depletion of energy substrates, and accumulation of metabolic by-products in cells. The essence of shock lies in the imbalance between inadequate oxygen supply to tissues and increased oxygen demand. The production of inflammatory mediators is a hallmark of shock. Therefore, restoring oxygen delivery, promoting efficient oxygen utilization, re-establishing the balance between oxygen supply and demand, and maintaining normal cellular function are key elements in the treatment of shock.
Classification
Shock is typically divided into five categories: hypovolemic (including hemorrhagic and traumatic), septic, cardiogenic, neurogenic, and anaphylactic shock. Among these, hypovolemic and septic shock are the most common types encountered in surgical practice.
Pathophysiology
A sharp reduction in effective circulating blood volume and tissue perfusion, as well as the production of inflammatory mediators, form the common pathophysiological basis of various types of shock. Conditions such as trauma, hemorrhage, and infection can directly result in reduced tissue perfusion. At the same time, these conditions can induce a cellular inflammatory response, eliciting a series of inflammatory reactions that further exacerbate the insufficiency of tissue perfusion, thereby promoting the progression of shock.
Changes in Microcirculation
Microcirculation, which accounts for 20% of the total circulating blood volume, undergoes corresponding changes during shock caused by inadequate circulating volume.
Phase of Microcirculatory Constriction
In the early stages of shock, a significant decrease in effective circulating blood volume leads to reduced circulating capacity and a drop in arterial blood pressure. At this stage, the body undergoes several pathophysiological changes to maintain relative circulatory stability. These include: activation of baroreceptor-mediated pressor reflexes in the aortic arch and carotid sinus; excitation of the sympathetic-adrenal axis leading to increased catecholamine release; and elevated secretion of renin-angiotensin. These responses result in increased heart rate and cardiac output. Selective vasoconstriction of small blood vessels in peripheral (skin, skeletal muscle) and visceral tissues (e.g., liver, spleen, gastrointestinal tract) redistributes circulating blood volume, prioritizing perfusion to vital organs such as the heart and brain. Contraction of the smooth muscle in small arteries, veins, and precapillary sphincters, influenced by hormones such as catecholamines, causes strong vasoconstriction and the opening of arterio-venous shunts. This increases both vascular resistance in the periphery and venous return to the heart. Constriction of precapillary sphincters and relative dilation of postcapillary sphincters aid tissue fluid reabsorption, partially compensating for blood volume loss. However, within the microcirculation, the contraction of precapillary sphincters leads to a "blood outflow only, no inflow" scenario, resulting in reduced blood volume and continued tissue hypoperfusion and hypoxia. At this stage, the removal of the causative factor and active resuscitation may lead to an easier reversal of shock.
Phase of Microcirculatory Dilation
If shock continues to progress, an excessive opening of arterio-venous shunts and direct capillary pathways in the microcirculation further exacerbates the pre-existing tissue perfusion deficit. Severe tissue hypoxia induces a shift to anaerobic metabolism, resulting in energy depletion, accumulation of lactic acid, and the release of vasodilatory mediators such as histamine and bradykinin. These substances directly relax precapillary sphincters, while the sensitivity of postcapillary sphincters remains relatively low, causing them to stay constricted. This leads to a "blood inflow only, no outflow" situation in the microcirculation. Consequently, blood stasis within the capillary network increases hydrostatic pressure, and along with enhanced capillary permeability, results in plasma leakage, hemoconcentration, increased blood viscosity, and further reductions in venous return and cardiac output. This ultimately decreases perfusion to vital organs such as the heart and brain, worsening the shock and advancing it to the dilation phase of microcirculation.
Phase of Microcirculatory Failure
If the condition continues to deteriorate, it progresses to irreversible shock. In this phase, blood stasis within the microcirculation, combined with increased viscosity, creates a hypercoagulable state under acidic conditions, predisposing red blood cells and platelets to aggregation and the formation of microthrombi. This may lead to disseminated intravascular coagulation (DIC). At this stage, the severe lack of blood perfusion and oxygen prevents cells from maintaining energy metabolism, causing lysosomal membranes to rupture. The release of various acidic hydrolases from lysosomes induces cellular autolysis, damaging surrounding cells and leading to widespread tissue damage, dysfunction of entire organs, and eventually, failure of multiple organs.
Metabolic Alterations
Anaerobic Metabolism and Metabolic Acidosis
When oxygen supply becomes insufficient to meet cellular oxygen demand, anaerobic glycolysis occurs. As oxygen availability decreases, lactic acid production increases. In cases of severe acidosis (pH < 7.2), cardiovascular responsiveness to catecholamines diminishes, leading to manifestations such as bradycardia, vasodilation, and reduced cardiac output. Additionally, the oxyhemoglobin dissociation curve shifts to the right, further impairing oxygen delivery to tissues.
Energy Metabolism Disorders
Trauma and infection place the body in a state of stress, activating the sympathetic-adrenal medulla and hypothalamic-pituitary-adrenal axis. This state elevates catecholamine and corticosteroid levels, suppressing protein synthesis and promoting protein catabolism to supply energy and provide raw materials for the synthesis of acute-phase proteins. Hormonal changes also enhance gluconeogenesis and inhibit glycolysis, leading to increased blood glucose levels.
Under stress conditions, proteins are consumed as substrates. When proteins with specialized functions, such as enzymes, are depleted, complex physiological processes cannot proceed properly, contributing to the development of multiple organ dysfunction syndrome (MODS). In addition, lipolysis is significantly enhanced during stress, becoming a primary energy source in critically ill patients.
Release of Inflammatory Mediators and Ischemia-Reperfusion Injury
Severe trauma, infection, and hemorrhage stimulate the excessive release of inflammatory mediators, triggering a "cascade-like" amplification of inflammatory responses. These mediators include interleukins, interferons, and vasodilators such as nitric oxide (NO). Reactive oxygen species (ROS) may induce lipid peroxidation and cell membrane rupture.
During inflammatory responses, endothelial cells regulate blood flow, leukocyte adhesion, and aggregation, thereby influencing the progression of inflammation. Neutrophils are the first cells activated during this response. Inflammatory mediators and extracellular ligands activate neutrophils, facilitating their migration to tissues. While polymorphonuclear neutrophils (PMNs) play an essential role in clearing infection sources, activated PMNs also mediate cytotoxic effects by releasing ROS, proteolytic enzymes, and vasoactive molecules. These substances can exacerbate tissue and cellular damage and may contribute to the progression of MODS associated with shock.
Metabolic acidosis and energy depletion further impair the barrier functions of various cell membranes. In addition to increased membrane permeability, dysfunction of ion pumps such as the Na+-K+ pump and calcium pumps is observed. This dysfunction results in abnormal distributions of intracellular and extracellular ions and fluids—for example, sodium and calcium ions accumulate within cells and cannot be expelled, while potassium ions remain outside the cells. This imbalance leads to hyponatremia, hyperkalemia, and the migration of extracellular fluid into cells, causing cell swelling and death. The influx of large quantities of calcium ions into cells not only activates lysosomes but also increases mitochondrial calcium concentrations, disrupting mitochondrial function.
When lysosomal membranes rupture, numerous hydrolases are released, causing autolysis of cells and tissue damage. Additionally, the production of toxic factors such as myocardial depressant factor (MDF) and bradykinin is observed. Mitochondrial membrane damage leads to the degradation of membrane lipids into toxic products such as thromboxane and leukotrienes. These processes result in mitochondrial swelling, disappearance of mitochondrial cristae, and impaired oxidative phosphorylation, consequently reducing energy production.
Secondary Organ Damage
Lungs
Hypoxia during shock causes damage to pulmonary capillary endothelial cells and alveolar epithelial cells, reducing surfactant levels. During resuscitation, the use of large amounts of stored blood containing microparticles may lead to pulmonary microcirculation embolism. This exacerbates alveolar collapse, atelectasis, pulmonary edema, and insufficient perfusion or occlusion in some pulmonary vessels. These changes result in increased pulmonary shunting and dead space ventilation, which, in severe cases, may lead to acute respiratory distress syndrome (ARDS). ARDS commonly occurs during the shock phase or within 48–72 hours after stabilization.
Kidneys
A drop in blood pressure and increased catecholamine secretion cause afferent arteriolar spasm and a reduction in effective circulating volume, significantly lowering glomerular filtration rate and resulting in oliguria. Renal blood flow redistributes during shock, favoring the medulla over the cortex, which may lead to ischemic necrosis of renal cortical tubules and acute kidney injury.
Brain
Decreased cerebral perfusion pressure and cerebral blood flow cause cerebral hypoxia. Ischemia, CO2 retention, and acidosis lead to cerebral cell swelling and increased vascular permeability, resulting in cerebral edema and elevated intracranial pressure. Severe cases may culminate in brain herniation.
Heart
Reduced coronary blood flow causes myocardial ischemia. The formation of microthrombi in the myocardial microcirculation can lead to focal myocardial necrosis. The myocardium, being rich in xanthine oxidase, is particularly vulnerable to ischemia-reperfusion injury.
Gastrointestinal Tract
The mesenteric blood vessels possess a high density of angiotensin II receptors, making them highly responsive to vasopressors. During shock, blood flow through the superior mesenteric artery may decrease by up to 70%. Insufficient mucosal perfusion results in hypoxic injury to the intestinal mucosa, impairing its mechanical and immune barrier functions. This dysfunction allows bacteria and their toxins to enter the systemic circulation via lymphatic or portal venous pathways, a phenomenon known as bacterial and endotoxin translocation. This contributes to gut-driven infections, further progression of shock, and multi-organ failure, which are among the critical factors leading to mortality in the late stages of shock.
Liver
Shock-induced ischemic and hypoxic injury to the liver disrupts its synthetic and metabolic functions. Furthermore, harmful substances from the gastrointestinal tract activate liver Kupffer cells, leading to the release of inflammatory mediators. Biochemical tests may reveal elevated levels of transaminases and bilirubin, indicating metabolic abnormalities. The impaired liver loses its detoxification and metabolic capabilities, resulting in endotoxemia that exacerbates existing metabolic disturbances and acidosis.
Throughout the progression of shock, the aforementioned pathophysiological changes interact in a cause-and-effect manner, forming a vicious cycle that accelerates cellular damage and the development of multi-organ dysfunction.
Clinical Manifestations
Based on the progression of shock, it can be divided into the compensatory phase and the decompensatory phase, also referred to as the early stage and the shock stage.
Compensatory Phase
This phase is characterized by symptoms such as nervousness, excitement or restlessness, pale skin, cold extremities, increased heart rate, narrowed pulse pressure, rapid breathing, and decreased urine output. If timely and appropriate treatment is provided during this period, shock may be effectively corrected. Otherwise, the condition may progress to the decompensatory phase of shock.
Decompensatory Phase
In this phase, the patient may show symptoms such as apathy, slow responses, confusion, or even coma. Cold sweat, cyanosis of the lips and extremities, a rapid and weak pulse, and progressively decreasing blood pressure may also occur. In severe cases, extensive skin and mucous membrane cyanosis, cold extremities, an unpalpable pulse, undetectable blood pressure, and oliguria or anuria can be observed. The presence of petechiae on the skin or mucosa or gastrointestinal bleeding indicates the condition has likely progressed to the stage of disseminated intravascular coagulation (DIC). Progressive respiratory distress, rapid pulse, agitation, and cyanosis that cannot be alleviated by conventional oxygen therapy may suggest the onset of acute respiratory distress syndrome (ARDS).
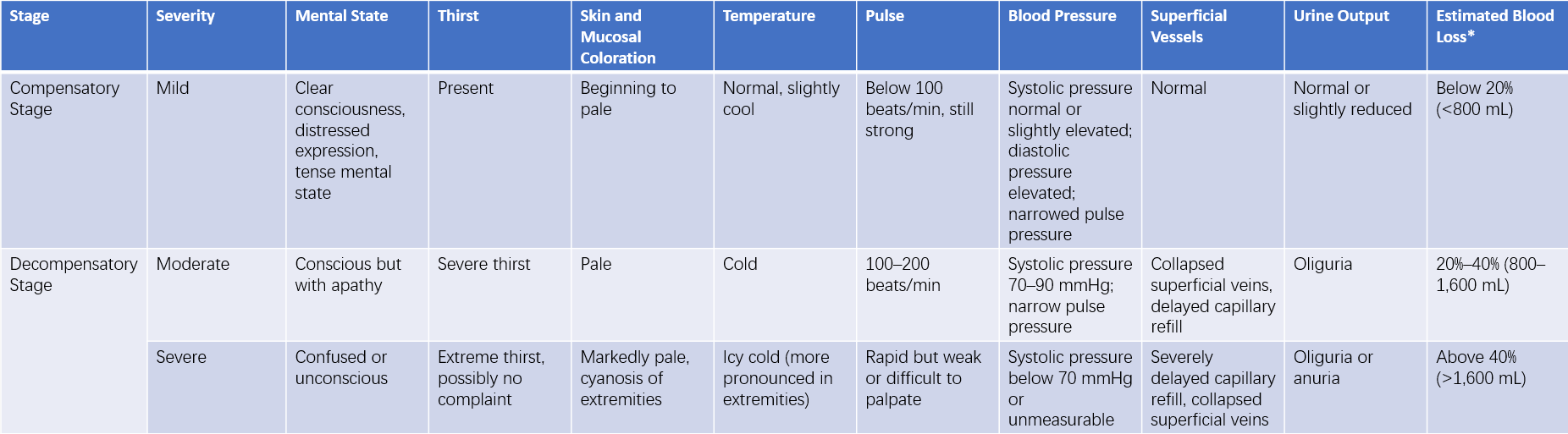
Table 1 Clinical manifestations and severity of shock
Note: *Estimated blood loss refers to adult hypovolemic shock.
Diagnosis
Early detection and accurate staging are critical for diagnosis:
Patients with severe trauma, massive bleeding, or severe infection should be assessed for the potential development of shock.
Clinical observations of symptoms such as sweating, excitement, increased heart rate, narrowed pulse pressure, or decreased urine output should raise suspicion of shock.
Markers of progression to the decompensatory phase of shock include symptoms such as apathy, slow responses, pale skin, rapid and shallow breathing, systolic blood pressure dropping below 90 mmHg, and oliguria or anuria.
Monitoring
Monitoring provides insights into the progression of the patient's condition and the effectiveness of treatment and serves as an objective basis for adjusting therapeutic strategies.
General Monitoring
Mental Status
Mental status reflects cerebral blood perfusion and overall circulatory status. A patient who remains alert and responds appropriately to external stimuli has generally achieved adequate circulating blood volume. In contrast, apathy, restlessness, delirium, drowsiness, or coma indicates cerebral dysfunction caused by compromised blood circulation.
Skin and Mucosal Temperature and Color
Skin temperature and coloration are indicators of peripheral blood perfusion. Warm extremities, dry skin, and rapid normalization of color after temporary ischemia (e.g., pressing and releasing a fingernail or lip) suggest recovery of peripheral circulation and improvement in shock. Conversely, cold extremities or delayed return of color may indicate the persistence of shock.
Blood Pressure (BP)
A systolic blood pressure of less than 90 mmHg or a pulse pressure of less than 20 mmHg is commonly indicative of shock. Recovery of blood pressure and an increase in pulse pressure are signs of improvement. However, blood pressure alone is not a sufficient indicator of shock severity, and should be interpreted in conjunction with other parameters.
Pulse Rate
Pulse rate is one of the key physiological indicators in shock monitoring:
During the early phase of shock, pulse acceleration often precedes changes in blood pressure, with a faster pulse yet normal blood pressure.
In the decompensatory phase of shock, both pulse rate increases and blood pressure decreases.
When shock improves, the pulse rate typically normalizes; however, blood pressure may remain normal or slightly below normal.
It is important to consider that vasopressor therapy or pre-existing cardiac conditions may affect the pulse rate and blood pressure, altering their clinical significance in assessing the severity of shock.
Urine Output (UO)
Urine output serves as an important indicator of renal blood perfusion. Oliguria often reflects unresolved shock. Urine output of less than 25 mL/h with increased urine specific gravity suggests persistent renal vascular constriction and inadequate perfusion. Normal blood pressure but persistently low urine output with reduced specific gravity may indicate acute kidney injury. Improvement in shock is typically accompanied by urine output exceeding 30 mL/h.
Specialized Monitoring
Specialized monitoring includes a variety of hemodynamic measurements:
Central Venous Pressure (CVP)
CVP reflects changes in pressure within the right atrium or thoracic segment of the central venous system, providing an indication of the relationship between overall blood volume and right heart function. The normal CVP range is 5–10 cmH2O. A CVP below 5 cmH2O indicates hypovolemia, while a CVP above 15 cmH2O suggests impaired cardiac function, excessive venoconstriction, or increased pulmonary circulation resistance. A CVP exceeding 20 cmH2O typically signals congestive heart failure.
Arterial Blood Gas Analysis (ABG)
Normal values for arterial oxygen partial pressure (PaO2) range from 80–100 mmHg, while arterial carbon dioxide partial pressure (PaCO2) typically falls between 36–44 mmHg.
During shock, inadequate pulmonary gas exchange can result in carbon dioxide retention, leading to a marked rise in PaCO2. Conversely, hyperventilation in patients without pre-existing pulmonary conditions may lower PaCO2. PaCO2 levels exceeding 45–50 mmHg often suggest alveolar ventilation dysfunction, while PaO2 levels below 60 mmHg that do not improve with pure oxygen inhalation may indicate early signs of acute respiratory distress syndrome (ARDS).
Monitoring pH and base excess (BE) helps assess acid-base balance during shock. Dynamic changes in these parameters offer insights into the progression of shock.
Arterial Blood Lactate Measurement
Insufficient tissue perfusion leads to anaerobic metabolism and hyperlactatemia. Lactate level monitoring aids in assessing the trends in shock progression and response to resuscitation. Elevated lactate levels are closely associated with poor prognosis, with persistent hyperlactatemia often indicating an increased risk of mortality.
In addition, measures such as DIC-related testing and cardiac output (CO) measurements using a pulmonary artery catheter (Swan-Ganz catheter) can provide further insights into hemodynamic status.
Currently, Pulse Indicator Continuous Cardiac Output (PiCCO) monitoring is widely used in critical care to track hemodynamic conditions in critically ill patients. PiCCO employs the thermodilution method to measure single cardiac output values and continuously derives cardiac output (PCCO) from analysis of arterial pressure waveforms. It can also calculate intra-thoracic blood volume (ITBV) and extravascular lung water (EVLW). Compared to conventional pulmonary artery catheter monitoring, PiCCO is less invasive, provides dynamic and continuous measurements, and is easier to perform.
Treatment
Treatment should focus on addressing the underlying causes of shock and the major physiological disturbances in its various stages. The main objectives are to restore perfusion and ensure sufficient oxygen delivery to tissues, with the overall aim of preventing multiple organ dysfunction syndrome (MODS).
Emergency Treatment
Emergency treatment involves managing primary injuries or conditions causing shock, such as immobilizing fractures, controlling severe bleeding, and maintaining airway patency. A semi-recumbent position with the head and torso elevated by 20°–30° and the lower limbs elevated by 15°–20° can help improve venous return. Early intravenous access should be established to administer medications for blood pressure stabilization. Oxygen therapy via nasal cannula or face mask should be initiated during the early phase. Maintaining the patient’s body temperature is crucial.
When managing critically ill or trauma patients, several principles should be followed:
- Ensuring airway patency.
- Promptly controlling active bleeding.
- Administering blood products and fluids for volume replacement during surgical bleeding control.
Volume Resuscitation
Volume resuscitation is critical for correcting tissue hypoperfusion and hypoxia caused by shock. The effectiveness of fluid replenishment can be evaluated based on monitored parameters, such as arterial blood pressure, urine output, and central venous pressure (CVP), combined with assessments of skin temperature, peripheral circulation, pulse, and capillary refill time. Crystalloids are currently the first-line fluid choice for volume resuscitation. Artificial colloids can be used in combination during large-volume fluid resuscitation, and component blood transfusion may be necessary.
For patients in shock, aggressive fluid resuscitation within the first "golden hour" following diagnosis is essential to rapidly restore optimal stroke volume, stabilize circulation, and improve oxygen delivery to tissues. This strategy, known as Early Goal-Directed Therapy (EGDT), represents a key approach in shock management.
Aggressive Management of Underlying Conditions
For shock caused by surgical conditions, underlying pathologies often require surgical intervention. Examples include visceral hemorrhage, bowel necrosis, gastrointestinal perforation, and abscesses. Effective shock treatment often necessitates timely surgery following the restoration of effective circulatory volume. In some cases, surgery may need to proceed simultaneously with anti-shock measures to avoid delaying life-saving interventions.
Correction of Acid-Base Imbalances
An acidic internal environment inhibits myocardial performance, vascular smooth muscle function, and renal activity. During the early phase of shock, excessive ventilation may also result in hypocapnia and respiratory alkalosis. Current practices in managing acid-base imbalances emphasize tolerating mild acidosis over alkalosis. The fundamental approach is to improve tissue perfusion and use alkaline agents appropriately, both in timing and dosage. Ensuring proper respiratory function is critical when using alkaline drugs, as inadequate ventilation may lead to CO2 retention and secondary respiratory acidosis.
Use of Cardiovascular Active Agents
The use of vasoactive medications during volume resuscitation can rapidly increase blood pressure and improve circulation, especially for patients with septic shock. Ideal vasoactive drugs can elevate blood pressure while improving blood flow to vital organs, including the heart, brain, kidneys, and intestines.
Vasoconstrictors
Common agents include dopamine, norepinephrine, and metaraminol. Dopamine is the most frequently used vasoactive drug and exhibits dose-dependent effects by stimulating α, β1, and dopamine receptors. At low doses (<10 μg/min·kg), dopamine primarily stimulates β1 and dopamine receptors, enhancing myocardial contractility, cardiac output, and vasodilation of renal and gastrointestinal vessels. At high doses (>15 μg/min·kg), it predominantly stimulates α receptors, increasing peripheral vascular resistance. For shock treatment, low doses are preferred to enhance cardiac function and dilate visceral vessels. When combined with other vasopressors, small doses of dopamine may be used to raise blood pressure without requiring dose escalation. Dobutamine exhibits stronger positive inotropic effects than dopamine, improving cardiac output and pump function. The combination of norepinephrine and dobutamine is particularly effective for septic shock. Norepinephrine, primarily an α receptor agonist with mild β receptor stimulation, increases myocardial contraction, vascular constriction, blood pressure, and coronary blood flow, although its duration of action is short. Metaraminol indirectly stimulates α and β receptors, with similar but weaker and shorter-lasting effects compared to norepinephrine (approximately 30 minutes).
Vasodilators
Vasodilators include α receptor antagonists (e.g., phentolamine and phenoxybenzamine) and anticholinergic drugs (e.g., atropine, anisodamine, and scopolamine). α receptor antagonists can counteract norepinephrine-induced small-vessel constriction, reduce microcirculatory stasis, and enhance left ventricular contractility. Anticholinergics are often used in the treatment of septic shock.
Positive Inotropes
Drugs that enhance myocardial contractility, such as dopamine, dobutamine, and cardiac glycosides like deslanoside, are commonly used. These drugs are often indicated when fluid volume is adequate but arterial pressure remains low, as determined by CVP monitoring.
The selection of vasoactive agents should be tailored to the patient's primary condition. In the early phase of shock, precapillary microvascular spasms are predominant, whereas in later stages, spasms primarily affect microvenules and small veins. Thus, vasodilators are often combined with volume replacement therapy. If necessary, vasoconstrictors may be used during volume expansion, but doses should be kept low and duration limited.
Treatment of DIC and Improvement of Microcirculation
For diagnosed disseminated intravascular coagulation (DIC), anticoagulants like heparin may be used. Additional therapies may include antifibrinolytic agents such as tranexamic acid or aminocaproic acid, as well as antiplatelet drugs like aspirin, dipyridamole, and low-molecular-weight dextran (dextran 40).
Application of Glucocorticoids and Other Medications
Glucocorticoids can be used for septic shock and other severe forms of shock. The main mechanisms include:
- Blocking α receptor stimulation, leading to vasodilation, reduced peripheral vascular resistance, and improved microcirculation.
- Protecting intracellular lysosomes and preventing lysosomal rupture.
- Enhancing myocardial contractility and cardiac output.
- Improving mitochondrial function and preventing leukocyte aggregation.
- Promoting gluconeogenesis to convert lactate into glucose, thereby alleviating acidosis.
To minimize potential side effects, glucocorticoids are generally recommended for early use in high doses but limited to a duration of less than 48 hours.
Continuous dynamic evaluation during shock resuscitation is essential. In addition to monitoring vital signs, increasing attention is being paid to parameters such as lactate and base excess, which are linked to tissue perfusion and metabolism. Lactate and base excess are considered reliable indirect indicators of hypoxia, tissue acidosis, and anaerobic metabolism, and are useful for predicting prognosis. Techniques such as CVP and PiCCO allow dynamic hemodynamic monitoring, providing insights into the recovery of effective circulating volume and facilitating timely treatment adjustments.