Peripheral nerve injuries can result in sensory and motor dysfunction. Without timely, accurate, and effective treatment, the prognosis is very poor and can lead to lifelong disability.
Applied Anatomy
Peripheral nerves are composed of nerve fibers. A nerve fiber consists of the extension of a neuron's cell body and is made up of an axon, a myelin sheath, and a Schwann sheath. The axon forms the central axis of the nerve fiber and contains microfilaments, microtubules, mitochondria, and axoplasm. The axon connects the nerve cell body to muscles and skin receptors, facilitating the conduction of information. The myelin sheath, composed of myelin lipids and proteins, wraps around the axon in segments, with interruptions called the nodes of Ranvier that prevent the spread of excitation. The Schwann sheath, composed of Schwann cells, serves as a pathway for nerve regeneration.
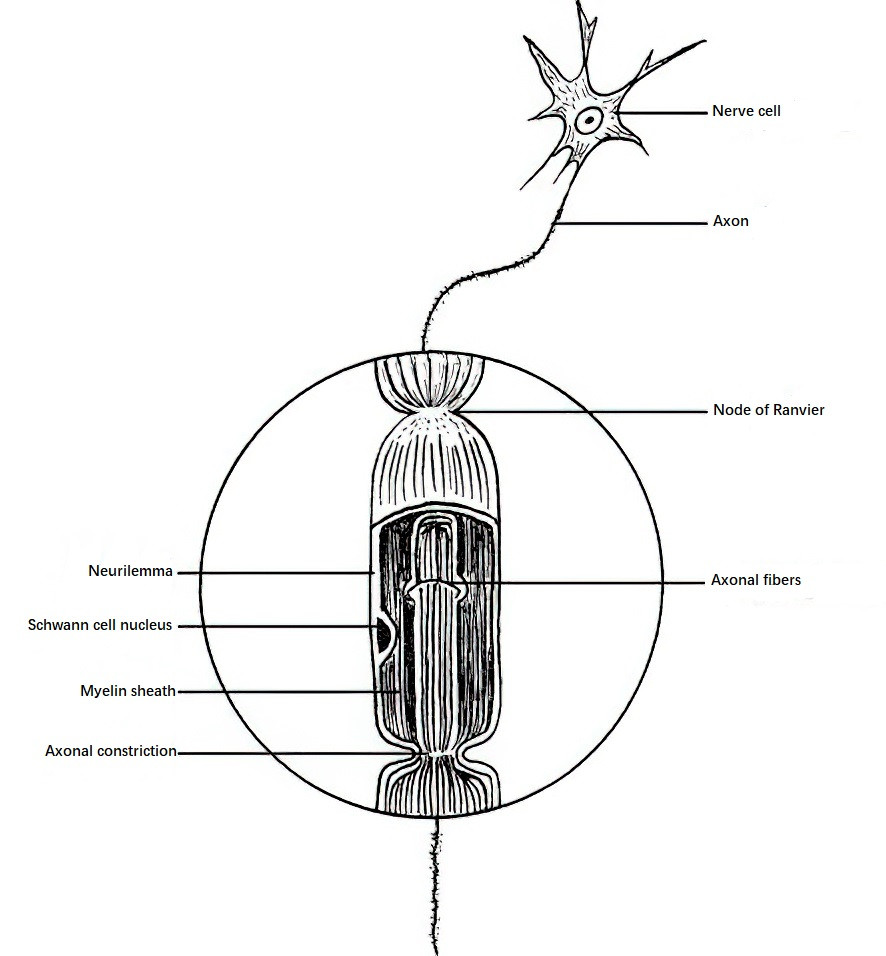
Figure 1 Nerve fiber structure
Classification of Nerve Injuries
Nerve injuries are classified based on the severity and nature of the damage. The Seddon classification is commonly used, dividing injuries into three categories:
Neuropraxia
This is characterized by temporary loss of sensory and motor function without structural changes to the nerve fibers. Function typically recovers spontaneously within a few days to weeks. It is often caused by mild traction or short-term compression.
Axonotmesis
The pathological feature is degeneration (Wallerian degeneration) or demyelination of the axon distal to the injury site. The endoneurial tube remains intact, enabling the axon to regenerate along the Schwann sheath to the distal target. Functional recovery is generally achievable. This type of injury is often caused by blunt trauma or sustained compression.
Neurotmesis
Complete nerve transection occurs, resulting in loss of nerve function. Function can only be restored through surgical repair.
Pathology and Regeneration
When a nerve is transected, pathological changes occur in nerve fibers, neuronal cell bodies, and target organs. The distal parts of the nerve fibers undergo Wallerian degeneration. Within hours of injury, structural changes occur in the distal axon and myelin sheath. Disintegration into small fragments is observed after 2–3 days, and phagocytic cells then proliferate to clear the debris by the 5th–6th day. Simultaneously, Schwann cells proliferate—peaking around the 3rd day and continuing for 2–3 weeks—to form hollow pathways (Schwann tubes) that allow regenerating axons from the proximal stump to grow into the distal segment. Similar changes occur in the proximal stump, but typically only within 1–2 nodes of Ranvier at the site of injury.
The neuronal cell body exhibits changes known as the axonal reaction, including cell body swelling and Nissl body dissolution or disappearance. The closer the injury site is to the cell body, the more pronounced the reaction is, which may lead to cell death in severe cases. The target organs (e.g., motor endplates, tactile corpuscles) also undergo degeneration, atrophy, or even disappearance.
Nerve regeneration is characterized by the emergence of several regenerating axonal sprouts from the proximal stump approximately 1 week after injury. If the two nerve ends are connected, the regenerating axons grow into the distal Schwann tube at a rate of 1–2 mm per day, eventually re-establishing function in the target organ. Schwann cells gradually surround the regenerating axons to form new myelin sheaths. If the nerve ends are not connected, the regenerating fibers from the proximal stump form a coiled, bulbous mass termed a pseudoneuroma. In the distal segment, Schwann cells and fibroblasts proliferate to form a neuroma. After surgical nerve repair, the process involves degeneration, regeneration, migration through the scar tissue, and target organ maturation, which takes a prolonged recovery period.
Clinical Presentation and Diagnosis
Motor Dysfunction
After nerve injury, the muscles innervated by the affected nerve undergo flaccid paralysis, leading to the loss of voluntary movement, muscle tone, and tendon reflexes. It is crucial to note that some joint movements may be compensated by other muscles. The strength of each muscle should be individually assessed for accurate evaluation. Muscle strength imbalance around the joint may cause characteristic deformities, such as wrist drop with radial nerve injuries above the elbow or claw hand with ulnar nerve injuries above the wrist. Over time, muscle atrophy occurs, and its severity and extent depend on the location, degree, and duration of the nerve injury.
Sensory Dysfunction
Skin sensation includes touch, pain, and temperature perception. Touch can be assessed with cotton, pain with pinpricks, and temperature with hot or cold stimuli. After nerve transection, skin sensation in the affected area is lost. Since sensory innervation has overlapping territories, sensory loss should be evaluated in the absolute supply zone of the nerve, such as the distal phalanges of the index and middle fingers for the median nerve and the little finger for the ulnar nerve. Partial injuries may manifest as reduced sensation or hypersensitivity. Sensory function tests are helpful in tracking nerve recovery, particularly two-point discrimination, which measures the ability to distinguish two points of simultaneous stimulation on the skin. In normal individuals, the two-point discrimination distance is 4–7 mm for the proximal phalanges and 3–5 mm for the distal phalanges. This can be assessed using calipers with two separate endpoints or specialized devices for two-point discrimination testing.
Autonomic Nervous Dysfunction
This primarily manifests as sympathetic nervous dysfunction. In the early stages, symptoms include vasodilation and cessation of sweat gland activity, leading to flushed skin, increased skin temperature, dryness, and absence of sweating. In later stages, vasoconstriction results in pale skin, reduced skin temperature, a subjective feeling of coldness, a smoothened skin texture, as well as thickened, ridged, curved, and slow-growing nails. Finger skin tactile tests and chemical methods to assess sweat gland function are helpful in determining whether a nerve injury has occurred and in evaluating recovery after injury. The absence of sweating indicates nerve damage, while the return of sweating indicates nerve function recovery; excessive sweating is often observed in the early stages of recovery.
Tinel’s Sign (Percussion Test)
A positive Tinel’s sign occurs when localized pressure or percussion over a nerve trunk produces a sensation of pinprick pain radiating into the nerve's distribution area. This indicates the site of nerve injury. When percussion is applied from the site of nerve repair toward the distal portion along the nerve trunk, a positive Tinel’s sign signals nerve recovery. This makes Tinel’s sign significant for diagnosing nerve injuries and evaluating functional recovery.
Electrophysiological Testing
Electromyography (EMG) and somatosensory evoked potentials (SEP) hold substantial value in identifying the location and severity of nerve injury, as well as monitoring nerve regeneration and functional recovery.
Treatment
Treatment Principles
The primary goal is the earliest possible restoration of nerve continuity.
Closed Injuries
These injuries often involve contusions or traction of the nerve, resulting in conduction dysfunction or axonal disruption. Most cases recover spontaneously. Observation for 3 months is generally recommended, during which necessary pharmacological and physical therapy may be administered. The evaluation involves Tinel’s sign testing and electromyography (EMG). If there is no functional recovery or if partial recovery plateaus without further progress, surgical exploration is usually indicated.
Open Injuries
The time to surgery depends on the nature, severity, and contamination of the injury. Common surgical approaches include:
- Primary Repair: Performed within 6–8 hours following the injury and suitable for clean, lacerated wounds where technical and equipment conditions are favorable.
- Delayed Repair: Conducted 2–4 weeks post-injury for cases where primary repair was not performed, provided the wound is not infected.
- Secondary Repair: Undertaken 2–4 months post-injury for cases involving infected wounds, gunshot injuries, or high-velocity blast injuries, where the extent and severity of damage need careful assessment.
For crush or avulsion injuries with irregular and unrepairable nerve endings and uncertain damage extent, the nerve ends can be fixed to surrounding tissues during the initial surgery. This prevents retraction and facilitates later-stage repair.
Surgical Techniques
Neurolysis
This technique involves the incision or removal of scar tissue surrounding or within the nerve to relieve compression, improve the environment for nerve regrowth, and restore blood supply, aiding in nerve recovery.
Neurorrhaphy (Nerve Suture)
Two main types of neurorrhaphy are commonly performed:
- Epineurial Suture: Suitable for mixed nerves containing both motor and sensory fascicles.
- Perineurial Suture: Applied to nerves with homogeneous fascicles.
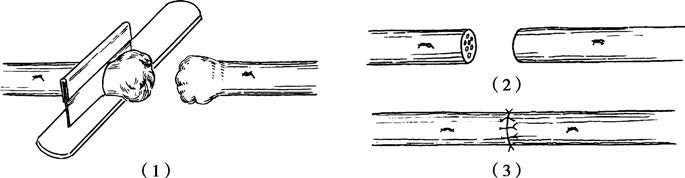
Figure 2 Epineurial suture
(1) Trimming the stump
(2) Preparing for suture
(3) Suturing the epineurium
Before suturing, the nerve ends are trimmed or the scarred edges are excised to expose healthy fascicles. Alignment of the two nerve ends is achieved by matching their external features, vascular orientation, and fascicular anatomy to avoid twisting or overlapping. Microsutures of 7-0 to 9-0 are typically used for epineurium or perineurium suturing. When tension exists, adjustments such as mobilizing the nerve ends, adjusting joint positioning, or performing nerve grafting are considered.
Nerve Transfer
When nerve defects cannot be repaired by tension adjustment, nerve grafting is performed. Donor nerves are typically superficial sensory nerves, with the sural nerve being the most commonly used. For large nerve trunks, multiple strands of grafted nerves are arranged in a cable-like configuration. For long gaps (≥10 cm), nerve grafting combined with vascular anastomosis may be required.
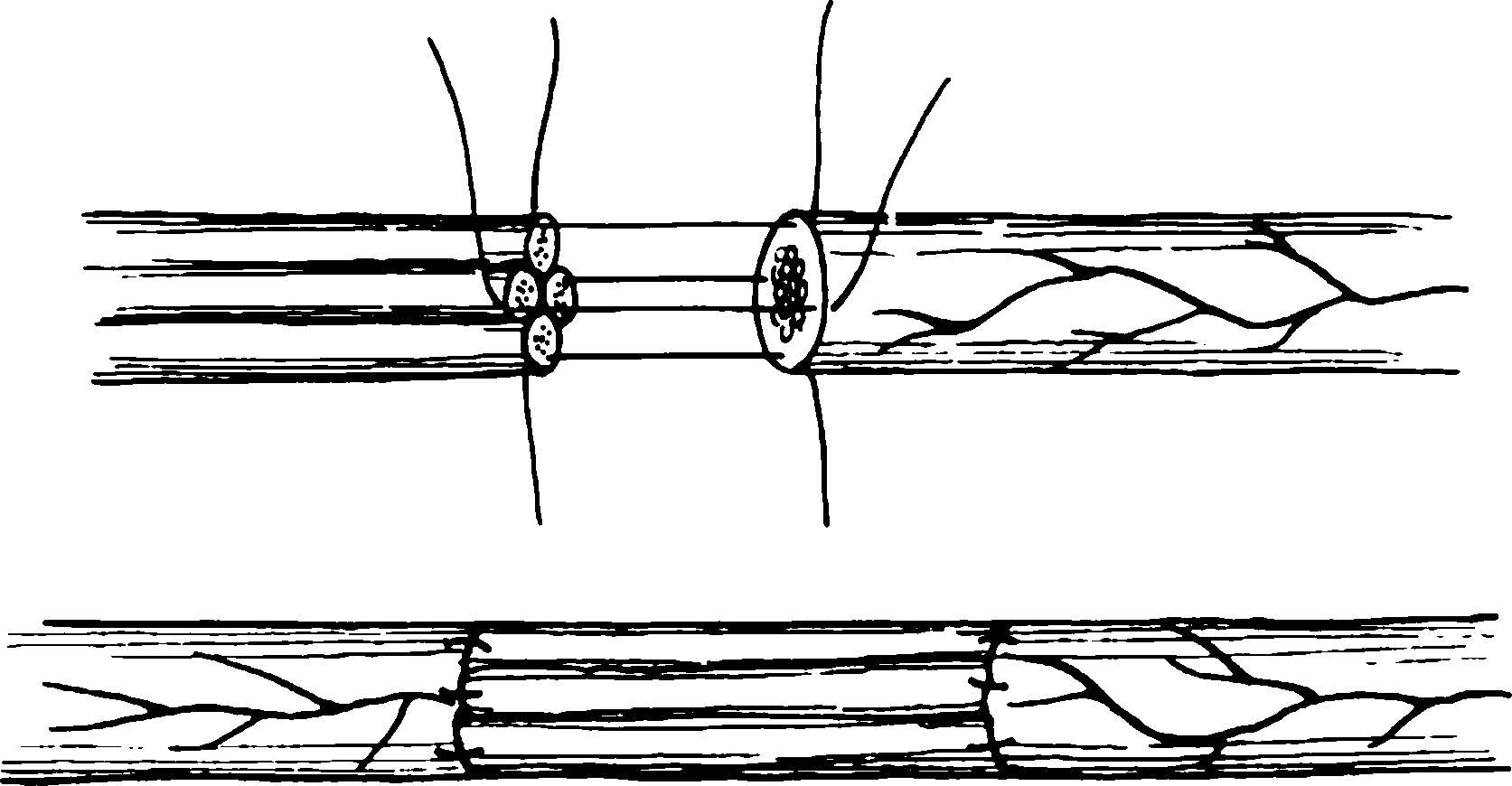
Figure 3 Nerve cable suture
Nerve Transposition
In cases of high-level nerve injuries that cannot be repaired, functionally less important nerves are redirected. The proximal end of the substitute nerve is connected to the distal end of the important damaged nerve to restore critical limb functions.
Nerve Implantation
When the distal nerve is damaged at its entry point into the muscle and cannot be sutured, the proximal nerve is divided into smaller fascicles, which are then implanted into the muscle tissue. This facilitates the formation of new motor endplates or reconnection with existing ones, partially restoring muscle function. Sensory nerves can similarly be implanted into subcutaneous tissue to form new sensory receptors, thereby restoring localized sensation.