Cushing syndrome (CS), initially described in 1932 by American neurosurgeon Harvey Cushing, is a condition caused by prolonged exposure to excessive glucocorticoids. The disorder is characterized by physiological and pathological changes resulting from adrenal overproduction of cortisol, leading to abnormalities in fat metabolism and distribution, protein metabolism (reduced synthesis, accelerated breakdown, and negative nitrogen balance), as well as increased gluconeogenesis and impaired glucose uptake and utilization. These processes result in various metabolic disturbances involving nutrients and electrolytes.
Etiology and Pathology
Cushing syndrome is classified into two main categories based on its underlying cause: ACTH-dependent and ACTH-independent.
ACTH-Dependent Cushing Syndrome (Corticotropin-Dependent Cushing Syndrome)
Cushing Disease
This accounts for 70%–80% of CS cases and is caused by excessive ACTH secretion from the anterior pituitary gland due to pituitary adenomas or functional abnormalities of the hypothalamus.
Ectopic ACTH Syndrome (Ectopic Corticotropic Syndrome)
This occurs when non-pituitary tumors secrete large amounts of ACTH or ACTH-like substances, leading to adrenal cortical hyperplasia and excessive cortisol production. Commonly associated tumors include small-cell lung cancer, thymomas, pancreatic neuroendocrine tumors, bronchial carcinoids, and medullary thyroid carcinomas.
ACTH-Independent Cushing Syndrome (Corticotropin-Independent Cushing Syndrome)
Cortisol-Secreting Adrenal Adenomas or Carcinomas
These tumors are the primary cause of ACTH-independent CS and account for 15% of cases. The excessive cortisol produced by adrenal tumors results in feedback inhibition of pituitary ACTH secretion, which may lead to atrophy of both the ipsilateral and contralateral normal adrenal cortex outside the tumor.
Adrenocorticotropin-Independent Macronodular Adrenal Hyperplasia (AIMAH)
This is a rare cause of CS, characterized by bilateral macronodular adrenal hyperplasia of varying size.
Clinical Manifestations
Central Obesity
Centripetal fat distribution is observed, manifesting as a “moon face,” “buffalo hump,” pendulous abdomen, short neck, and muscle atrophy in the extremities.
Skin Symptoms
The skin becomes thin, with the appearance of purple striae (commonly on the lower abdominal wall, inner thighs, and underarms), acne, and excess hair growth.
Hypertension and Hypokalemia
Blood pressure is usually moderately elevated, with increases in both systolic and diastolic pressures.
Gonadal Dysfunction
Females may exhibit virilization, such as acne, excess body hair, and the growth of a small mustache. Males may experience reduced libido and erectile dysfunction. In children, CS is characterized by generalized obesity and growth retardation.
Osteoporosis
Patients often report back pain, with potential height loss due to pathological fractures involving the ribs, thoracic spine, or lumbar spine.
Psychiatric Symptoms
Symptoms include insomnia, memory impairment, and difficulty concentrating. Severe cases may present with schizophrenia-like psychiatric disorders.
Increased Risk of Bacterial or Fungal Infections
Auxiliary Examinations
Laboratory Tests
Plasma and Urinary Free Cortisol Measurements
Blood samples are taken at 8:00 AM, 4:00 PM, and midnight to measure plasma cortisol levels, showing an increase in cortisol without the normal diurnal rhythm. Urinary free cortisol levels are also elevated.
Plasma ACTH Measurement
After confirming hypercortisolism, plasma ACTH levels are assessed to explore the underlying cause. In cases of cortisol excess caused by adrenal tumors, ACTH levels are decreased. In patients with adrenal hyperplasia resulting from Cushing disease, CRH-secreting tumors, or ectopic ACTH syndrome, ACTH levels are elevated.
Test-Based Examinations
Low-Dose Dexamethasone Suppression Test
At midnight, 1 mg of dexamethasone is orally administered. Plasma free cortisol is measured at 8:00 AM on the day of administration and the following morning. A reduction in cortisol levels by more than 50% compared to baseline suggests normal findings or simple obesity, while CS patients show little to no reduction.
High-Dose Dexamethasone Suppression Test
At midnight, 8 mg of dexamethasone is orally administered. Plasma free cortisol is measured at 8:00 AM on the day of administration and the following morning. A decrease of more than 50% compared to the baseline value suggests Cushing disease, while patients with adrenal tumors or ectopic ACTH syndrome exhibit minimal change in cortisol levels.
Localization Studies
Ultrasound (US)
Although simple and non-invasive, ultrasound has limited sensitivity but is valuable for the localization of adrenal tumors.
CT Scanning
More than 99% of adrenal adenomas and hyperplasia cases can be diagnosed. Adrenal adenomas are usually over 2 cm in diameter, and cortisol-secreting adenomas may exhibit higher CT attenuation values compared to aldosterone-producing adenomas.
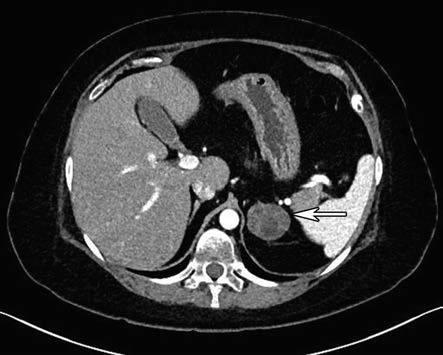
Figure 1 CT image of left adrenal cortical tumor
MRI Scanning
MRI has a sensitivity of approximately 95% for detecting adrenal tumors and is helpful in distinguishing adrenal adenomas from adrenocortical carcinomas. For Cushing disease, thin-slice coronal MRI of the sellar region helps identify pituitary lesions.
Diagnosis
The diagnosis of Cushing syndrome is primarily based on an assessment of the patient’s typical clinical features. Initial screening often involves adrenal ultrasound; if abnormalities in adrenal morphology are detected, further confirmation can be obtained through CT scanning. For suspected Cushing disease, pituitary MRI is recommended. Patients with suspected ectopic ACTH syndrome require additional imaging of the chest, neck, and anterior mediastinum to exclude tumors from other sources. Measurement of cortisol and ACTH levels in blood and urine, combined with relevant test-based examinations, provides a more comprehensive diagnostic evaluation to confirm CS.
Treatment
Surgical Treatment
Cushing Disease
For cases involving pituitary or hypothalamic lesions, the pituitary adenoma is excised using a transsphenoidal approach under surgical microscopy performed by a neurosurgeon.
Adrenal Adenoma or Carcinoma
Laparoscopic adrenalectomy is the preferred treatment due to its minimal invasiveness, rapid recovery, and good therapeutic outcomes. For larger tumors or suspected adrenocortical carcinomas, robotic-assisted or open surgical approaches are employed. Cortisol hypersecretion from the tumor suppresses ACTH production by the pituitary, leading to atrophy of the contralateral adrenal cortex; perioperative replacement of glucocorticoids is necessary to prevent adrenal crises.
Nodular Adrenal Hyperplasia
Bilateral adrenalectomy may be performed, involving total resection on one side and subtotal resection on the opposite side. To avoid lifelong hormone replacement, unilateral adrenalectomy (targeting the more hyperplastic side) using laparoscopic techniques has become the recommended approach. Postoperative cortisol levels should be regularly monitored to determine the timing and surgical strategy for the contralateral side.
Ectopic ACTH Syndrome
Resection of the primary tumor is performed to eliminate the source of ACTH secretion. If the tumor cannot be located or excised, bilateral adrenalectomy or partial adrenal tissue preservation may be considered to reduce symptoms.
Medication
Mitotane acts directly on the adrenal cortex to suppress cortisol synthesis and has cytotoxic effects on adrenal tumor tissue, making it suitable for adrenocortical carcinoma.
Aminoglutethimide inhibits the conversion of cholesterol to pregnenolone, thereby suppressing corticosteroid synthesis. Some patients may develop symptoms of adrenal insufficiency during treatment. In cases of postoperative recurrence or inoperable adrenocortical carcinoma, medication serves as an adjunctive treatment option for managing symptoms.