Prostate cancer is a common malignant tumor in elderly men, with significant regional and racial differences in incidence. Globally, prostate cancer has the highest incidence among male solid malignancies in Western countries, while incidence rates in Asia are much lower than in Europe and North America.
Etiology
The exact causative factors of prostate cancer remain unclear; however, it is believed to be associated with race, genetics, environmental influences, diet, obesity, and sex hormones. There are significant racial differences in high-frequency gene mutations and single nucleotide polymorphisms (SNPs) associated with prostate cancer risk. Having a first-degree relative with prostate cancer increases an individual's risk by more than double, and patients with a positive family history tend to be diagnosed approximately 6–7 years earlier. Excessive consumption of red meat and dairy products has been suggested to be a risk factor for prostate cancer. Research indicates that androgens, such as dihydrotestosterone, play an important role in prostate cancer development.
Pathology
More than 95% of prostate cancers are acinar adenocarcinomas, originating from glandular epithelial cells. Rare subtypes include intraductal carcinoma, ductal adenocarcinoma, squamous cell carcinoma, and neuroendocrine tumors. Prostate cancer predominantly arises in the peripheral zone and often has multifocal origins. The degree of differentiation varies widely. Pathological features include disorganized glandular structures, nuclear atypia, and infiltrative growth patterns, with nuclear atypia being a key diagnostic criterion. High-grade prostatic intraepithelial neoplasia (HGPIN) is considered a potential precursor lesion of prostate cancer.
Prostate cancer is histologically graded based on the degree of glandular differentiation and tumor growth patterns, with the Gleason grading system being the most widely used. This system is correlated with treatment outcomes and prognosis. In the Gleason grading system, tumor components are classified into primary and secondary grades based on their proportion, with each grade ranging from 1 to 5. The Gleason score (GS) is the sum of the primary and secondary grades, ranging from 2 to 10. Patients are categorized into low-risk (GS 6), intermediate-risk (GS 7), and high-risk (GS ≥8) groups, with higher scores indicating worse prognosis.
Prostate cancer staging is typically performed using the TNM staging system, which is an effective tool for disease assessment and provides a basis for treatment decisions. Prostate cancer with a primary tumor stage of ≤T2, without lymph node or distant metastasis, is classified as organ-confined prostate cancer. Prostate cancer with a primary tumor stage of ≥T3 or with regional lymph node involvement but no distant metastasis is classified as locally advanced prostate cancer.
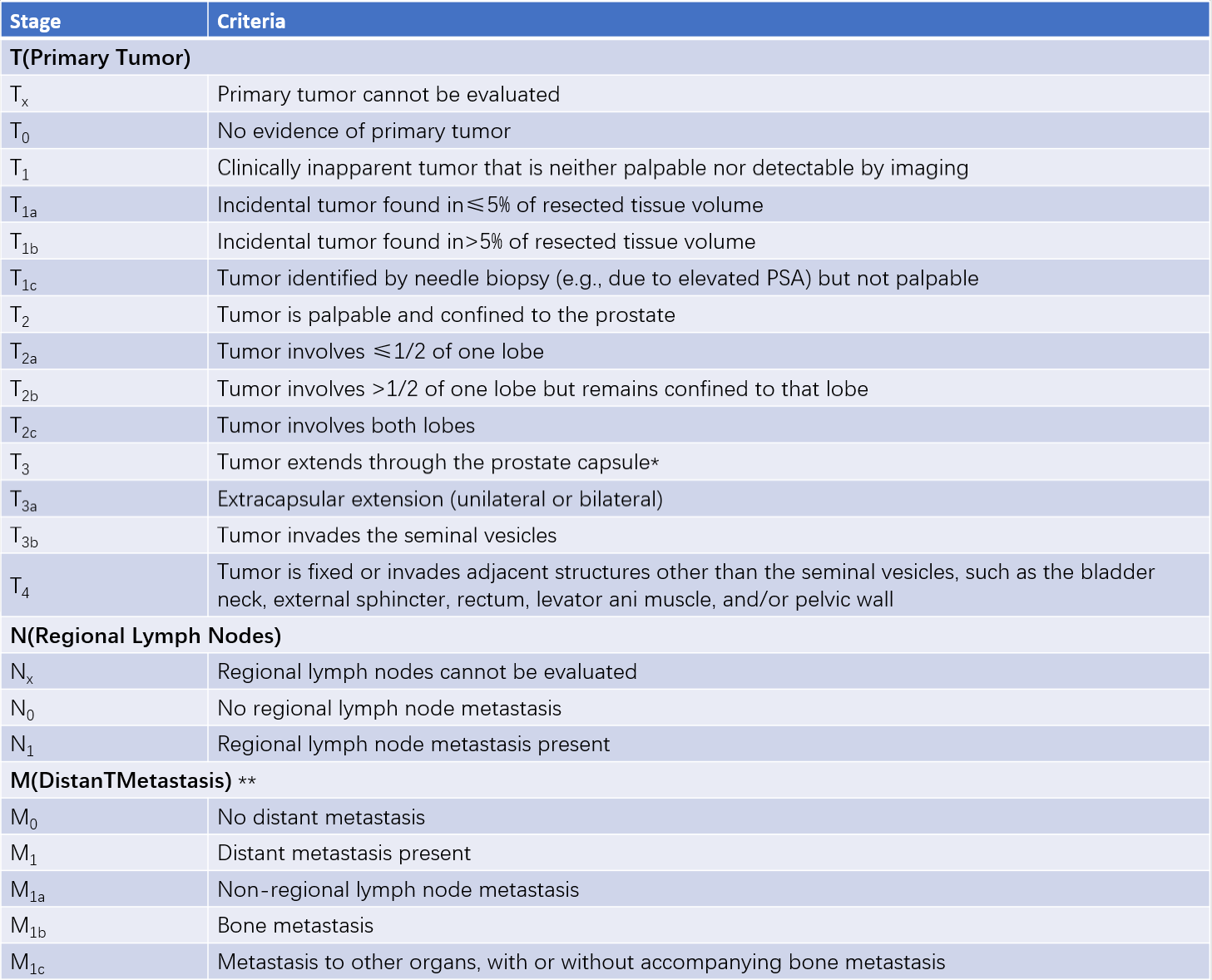
Table 1 TNM staging for prostate cancer (AJCC, 2017)
*Note:
Tumor involvement of the prostatic apex or capsule without capsular penetration is classified as T2, not T3.
**For cases with multiple metastases, the stage reflects the most advanced site of disease.
Clinical Manifestations
Prostate cancer predominantly affects elderly men. Most early-stage prostate cancers are asymptomatic and are often detected incidentally through physical examinations or pathological analysis following surgeries performed for other conditions, such as benign prostatic hyperplasia (BPH). As the tumor grows, it may cause lower urinary tract obstruction symptoms, such as increased urinary frequency, urgency, difficulty urinating, urinary retention, or incontinence. Tumor invasion of the urethra or bladder neck may result in gross hematuria.
Prostate cancer can metastasize via hematogenous or lymphatic spread, or through direct invasion of adjacent organs (e.g., seminal vesicles, bladder). The most common metastatic sites are lymph nodes and bones; other sites include the lungs, liver, brain, and adrenal glands. Bone metastases can manifest as bone pain, symptoms of spinal cord compression, and pathological fractures. Other symptoms of advanced prostate cancer include anemia, fatigue, lower limb edema, and bowel obstruction. In some cases, patients seek treatment primarily for symptoms caused by metastases, with minimal local symptoms, increasing the likelihood of misdiagnosis.
Diagnosis
The typical diagnostic approach for prostate cancer involves screening suspected cases through symptoms, physical examination, laboratory tests, and imaging studies, followed by histopathological confirmation with prostate biopsy.
Physical Examination
Digital rectal examination (DRE) can reveal prostate cancer nodules that are often harder than normal glandular tissue. However, in early-stage cancer or when the tumor originates in regions such as the transition zone, DRE findings are often unremarkable.
Laboratory Tests
Prostate-specific antigen (PSA) is an important serum marker for prostate cancer, with a normal reference range of 0–4 ng/ml. PSA levels are often elevated in prostate cancer and generally correlate with tumor burden.
Imaging Studies
Transrectal ultrasound (TRUS) was conventionally used for prostate cancer diagnosis, but early-stage prostate cancer often does not produce detectable abnormalities through this method. Multiparametric MRI (mpMRI) has high sensitivity and specificity for diagnosing prostate cancer and can provide an initial assessment of local tumor invasion and pelvic lymph node metastases. However, mpMRI is expensive and time-consuming. Typical mpMRI findings for prostate cancer include low-signal intensity on T2-weighted images, diffusion restriction with high signal intensity on DWI, low signal on ADC maps, and irregular enhancement on contrast imaging.
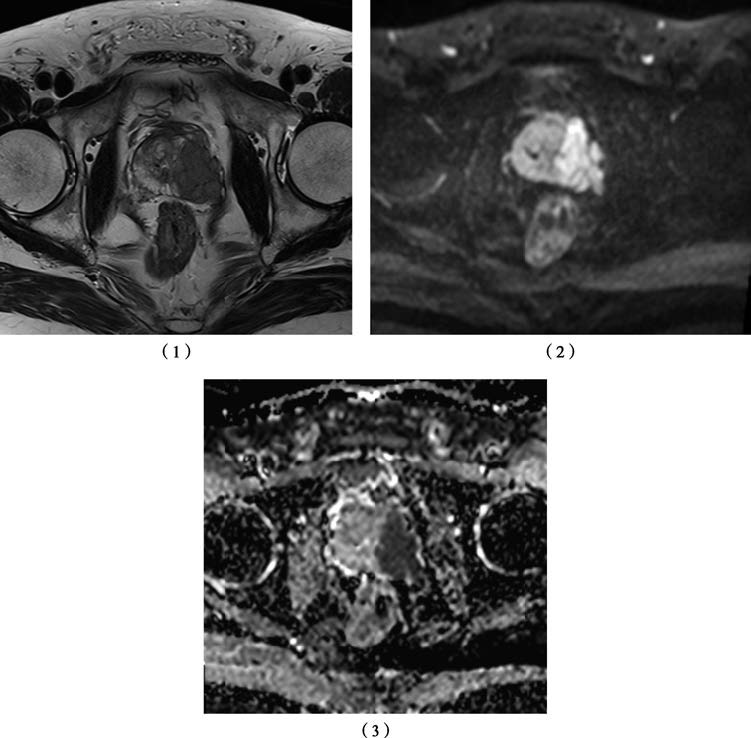
Figure 1 MRI scans of the prostate
1, T2-Weighted Image: Depicts a low-signal nodule in the left prostate lobe, extending through the capsule, corresponding to primary tumor staging T3a.
2, Diffusion-Weighted Image (DWI): Shows restricted diffusion in the prostate tumor with high signal intensity.
3, Apparent Diffusion Coefficient (ADC) Map: Shows the tumor as a low-signal lesion.
When prostate cancer metastasizes to bone, it typically produces osteoblastic lesions, which can be detected via whole-body skeletal scintigraphy or CT/MRI. PET/CT using radiolabeled prostate-specific membrane antigen (PSMA) offers higher accuracy for diagnosing primary tumors, lymph nodes, visceral, and bone metastases but is costly. Imaging is also useful for identifying complications of advanced-stage prostate cancer; for example, IVU or CTU can detect bladder invasion or ureteral obstruction leading to hydronephrosis.
Prostate Biopsy
Prostate biopsy is the primary method for confirming the pathological diagnosis of prostate cancer. It is typically performed under TRUS guidance.
Treatment
Organ-confined prostate cancer can be effectively treated with radical surgery or radiotherapy, and in some cases may even be clinically cured. Some tumors grow slowly, allowing low-risk elderly patients to consider active surveillance (AS) and pursue further treatment only if disease progression occurs.
Locally advanced prostate cancer can be managed with surgery or a combination of multidisciplinary treatment, often based on radiotherapy. For patients with locally advanced or metastatic prostate cancer who are not suitable for surgery, palliative treatment based on endocrine therapy is often employed to extend survival and improve quality of life.
Surgical Treatment
Radical prostatectomy is one of the most effective treatments for organ-confined and select locally advanced prostate cancers. The procedure involves complete removal of the prostate and seminal vesicles, with lymphadenectomy performed as indicated by the patient’s risk stratification and lymph node involvement. Surgery can be performed using conventional open methods, laparoscopic approaches, or robot-assisted laparoscopic techniques.
Radiotherapy
Radiotherapy includes definitive radiotherapy, postoperative adjuvant radiotherapy, and palliative radiotherapy. For organ-confined tumors, definitive radiotherapy can achieve near-curative outcomes, with 5- to 10-year disease-free survival rates similar to those of radical prostatectomy. Adjuvant radiotherapy is used for patients at high risk of recurrence or metastasis postoperatively, aiming to eliminate residual tumor in the tumor bed or lymph nodes. Palliative radiotherapy is primarily used to treat bone metastases in prostate cancer to alleviate pain.
Endocrine Therapy
Androgens are closely associated with the development and progression of prostate cancer. Endocrine therapy includes androgen deprivation therapy (ADT) and anti-androgen therapy. ADT inhibits prostate cancer growth by reducing androgen levels and can be achieved through surgical or pharmacological methods. Surgical castration involves bilateral orchiectomy, while pharmacological castration disrupts the hypothalamic-pituitary-gonadal axis to suppress testosterone production. The medications used include luteinizing hormone-releasing hormone (LHRH) agonists (e.g., goserelin, leuprolide) and LHRH antagonists (e.g., degarelix).
Anti-androgen drugs block androgen receptor activity, thereby inhibiting tumor cell growth. Common agents include bicalutamide and flutamide, while newer anti-androgen agents (e.g., apalutamide, enzalutamide) and androgen synthesis inhibitors (e.g., abiraterone) have shown improved clinical efficacy when combined with ADT.
Prostate cancer is diagnosed as castration-resistant prostate cancer (CRPC) when PSA levels increase or clinical progression occurs despite ADT. The time to progression to CRPC varies from a few months to several years depending on tumor aggressiveness and treatment approaches.
Chemotherapy
Chemotherapy is an important treatment modality for metastatic or castration-resistant prostate cancer. Common agents include docetaxel.
Other Therapies
Emerging physical treatments such as cryotherapy and high-intensity focused ultrasound (HIFU) have shown some ability to control prostate cancer lesions, though their long-term efficacy and target populations remain uncertain.
Prognosis
Prognosis for prostate cancer patients is highly variable. Some forms of prostate cancer pose very low progression risks and are considered “clinically insignificant.” The 23-year cancer-specific survival rate for patients with organ-confined, low- to intermediate-risk prostate cancer after radical therapy is 80.4%, while the 7-year cancer-specific survival rate for high-risk organ-confined cases is 93%. In contrast, the 5-year overall survival rate for metastatic prostate cancer patients is only 40%–52%.