Bladder tumors rank as the second most common malignancy in the urinary system and male genital system. The vast majority originate from epithelial tissues, with more than 90% being urothelial carcinomas. Squamous cell carcinoma and adenocarcinoma each account for 2%–3%. Tumors of mesenchymal origin make up 1%–5%, most commonly sarcomas such as rhabdomyosarcoma, which are more frequently seen in children. This section primarily discusses bladder cancer originating from epithelial tissues.
Etiology
The exact causes of bladder cancer remain unclear, but several major risk factors have been identified:
Smoking
Smoking is the most significant risk factor, accounting for approximately 50% of bladder cancer cases. It increases the risk of developing bladder cancer by 2–3 times, likely due to the presence of aromatic amine derivatives in cigarettes. The risk decreases after cessation of smoking.
Prolonged Exposure to Industrial Chemicals
Prolonged exposure to chemicals used in industries such as dye, leather, rubber, plastic, and paint significantly increases the risk of bladder cancer. Established carcinogens include benzidine, β-naphthylamine, and 4-aminobiphenyl.
Chronic Bladder Infections and Long-Term Irritation by Foreign Bodies
Conditions such as bladder stones, bladder diverticula, schistosomiasis infection, or chronic indwelling catheters increase the risk of bladder cancer, with squamous cell carcinoma being most commonly associated.
Others
Risk factors also include the use of cyclophosphamide, pioglitazone, conventional medicines containing aristolochic acid, hair dyes, and pelvic radiation therapy.
Pathology
The pathology primarily involves tumor histological grading, growth patterns, and depth of invasion, with histological grading and depth of invasion having the most significant impact on prognosis.
Histological Grading
The WHO grading systems for urothelial tumors of the bladder are widely used, specifically the WHO 1973 and WHO 2016 grading systems. The WHO 1973 system classifies tumors based on the degree of cellular differentiation into three grades: well-differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3). The WHO 2016 system includes three categories: papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade urothelial carcinoma (LG), and high-grade urothelial carcinoma (HG). The two grading systems are distinct and cannot be directly correlated.
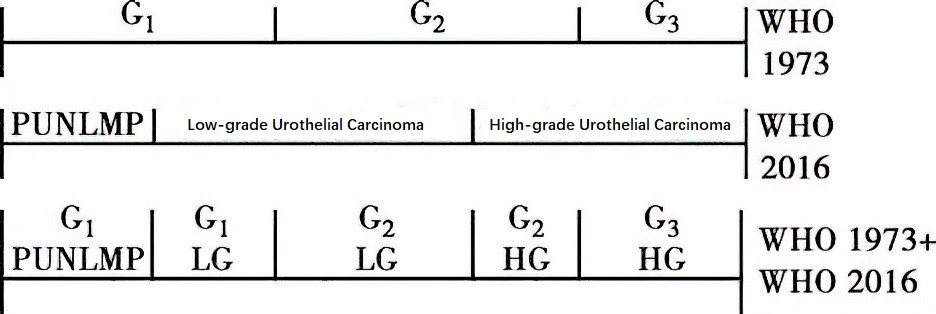
Figure 1 Grading systems for urothelial carcinoma (WHO 1973 and WHO 2016)
Patterns of Growth
Bladder cancer can manifest as carcinoma in situ (CIS), papillary carcinoma, or invasive carcinoma. CIS is confined to the mucosa, lacks papillary formations, and does not invade the basement membrane but is strongly associated with muscle-invasive disease. Urothelial carcinomas are typically papillary in form, with high-grade variants often exhibiting invasive potential. Different growth patterns may coexist within the same tumor.
Depth of Invasion
The depth of tumor invasion into the bladder wall, along with regional lymph node involvement and systemic metastasis, is assessed using the 2017 TNM staging system. This is one of the most valuable prognostic indicators. Clinically, tumors classified as Tis, Ta, or T1 are referred to as non-muscle-invasive bladder cancer (NMIBC), whereas T2 and higher stages are classified as muscle-invasive bladder cancer (MIBC). Although CIS is a type of non-muscle-invasive bladder cancer, it is generally poorly differentiated and carries a high risk of muscle invasion.
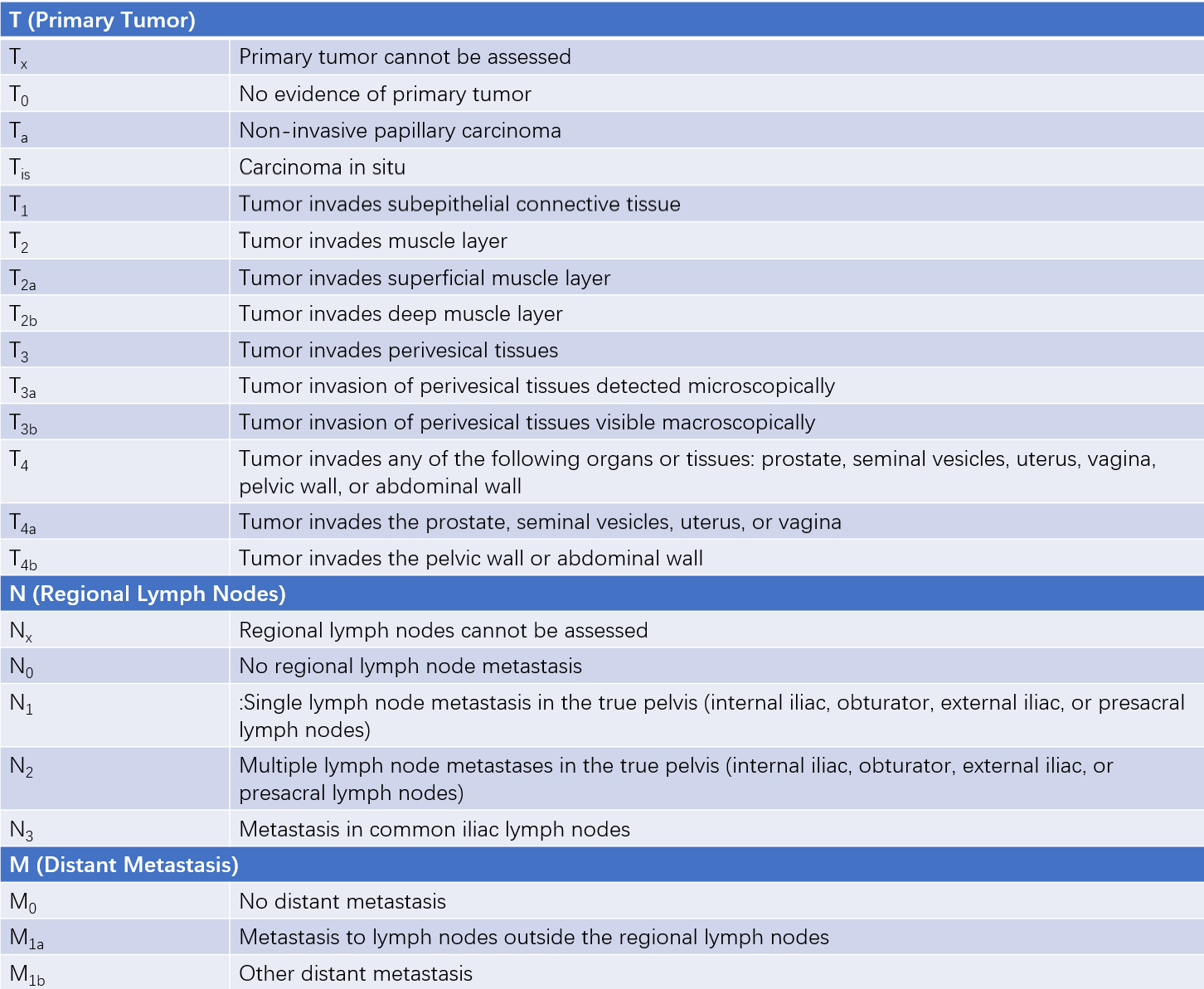
Table 1 TNM staging for bladder cancer (AJCC, 2017)
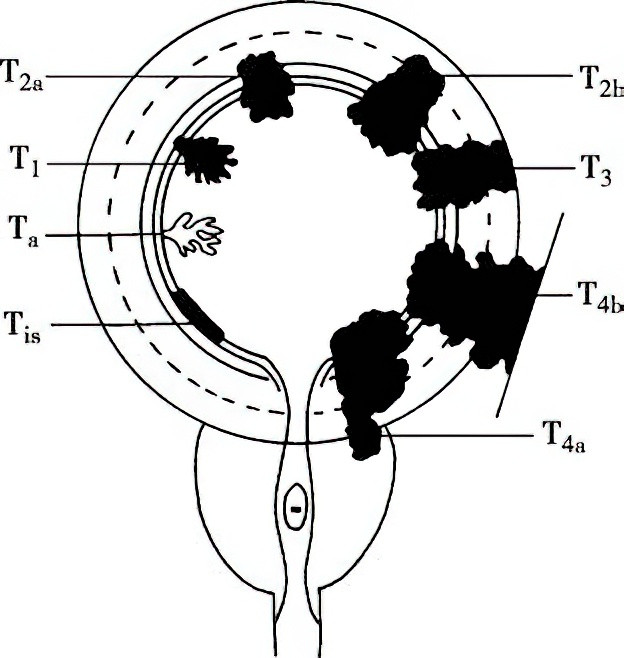
Figure 2 Depth of local invasion in bladder cancer
Recurrence, Progression, and Metastasis
Bladder cancer has a high recurrence rate, with non-muscle-invasive bladder cancer recurring in 50%–70% of cases. Approximately 15% of such tumors may progress to muscle-invasive bladder cancer after recurrence. Tumors that invade the bladder wall can breach the serosal layer and involve adjacent organs. Lymph node metastasis, particularly to pelvic lymph nodes such as the obturator and peri-iliac lymph nodes, is the most common early route of dissemination. Hematogenous metastasis typically occurs in the late stages, commonly affecting the bones, lungs, liver, and adrenal glands. Seeding metastasis can occur in areas such as abdominal incisions, the prostate fossa after bladder removal, and the abdominal cavity.
Clinical Manifestations
The majority of cases occur in individuals aged 50–70 years, with a male-to-female ratio of approximately 3–4:1. Hematuria is the most common symptom of bladder cancer. Intermittent, painless, gross hematuria throughout urination is the initial symptom in 80%–90% of patients. This may resolve spontaneously or temporarily, giving the false impression of "improvement" or "cure" and delaying treatment. Occasionally, hematuria may only be microscopic. The amount of bleeding does not always correlate with tumor size, number, or degree of malignancy. In some cases, the initial symptoms include urinary frequency, urgency, or dysuria, often associated with diffuse carcinoma in situ or muscle-invasive bladder cancer. A small proportion of patients are asymptomatic, with bladder tumors identified incidentally during health examinations.
Tumors located in the bladder trigone or neck may cause bladder outlet obstruction, leading to urinary retention or difficulty urinating. Tumors involving the ureters may result in hydronephrosis and present with flank pain. Extensive pelvic invasion or metastasis may lead to symptoms such as sacral or lumbosacral pain, lower limb edema, anemia, and weight loss. Bone metastasis can manifest as bone pain. Squamous cell carcinoma is often associated with chronic irritation from calculi or infections and may be accompanied by bladder stones.
Diagnosis
In middle-aged and elderly individuals, the presence of painless gross hematuria or recurrent microscopic hematuria detected through routine urinalysis raises suspicion of urinary tract tumors, with bladder cancer being the most common. The following diagnostic methods assist in confirmation:
Urine Cytology and Fluorescence In Situ Hybridization (FISH)
Tumor cells can often be identified in fresh urine sediment, making urine cytology one of the primary methods for bladder cancer diagnosis and postoperative follow-up. The sensitivity of urine cytology for bladder cancer ranges from 13% to 75%, while its specificity ranges from 85% to 100%. Low-grade tumor cells can be difficult to differentiate from normal urothelial cells or morphologically altered cells caused by inflammation or stones.
Urine FISH detects urothelial tumors by using fluorescently labeled probes to identify aneuploidy of chromosomes 3, 7, 17, and the 9p21 locus in exfoliated cells. Due to the fact that genetic abnormalities in tumors often occur earlier than morphological changes, urine FISH demonstrates higher diagnostic sensitivity than urine cytology, though its specificity is slightly lower.
Urinary Tumor Marker Testing
Testing for urinary DNA methylation has emerged in recent years as a marker for urothelial tumors, showing advantages in diagnosing early-stage, small-scale, and recurrent tumors. Other markers include nuclear matrix protein 22 (NMP22) and ImmunoCyt.
Imaging Studies
Ultrasound, being simple and convenient, effectively detects tumors larger than 0.5 cm in diameter and is often a first-line diagnostic method. Intravenous urography (IVU) and CT urography (CTU) can reveal filling defects for larger tumors and assess whether tumors are present in the renal pelvis or ureters, as well as the effects of bladder tumors on the upper urinary tract. Findings such as hydronephrosis or poor renal enhancement suggest tumor invasion of the ipsilateral ureteral orifice.
CT and MRI help evaluate the depth of tumor invasion into the bladder wall, lymph node involvement, and distant visceral metastases. MRI offers higher soft tissue contrast and resolution compared to CT, with superior sensitivity and specificity for diagnosing muscle-invasive bladder cancer. 18F-FDG PET-CT provides early detection of lymph node metastasis in bladder cancer. Radionuclide bone scanning is useful for assessing bone metastasis.
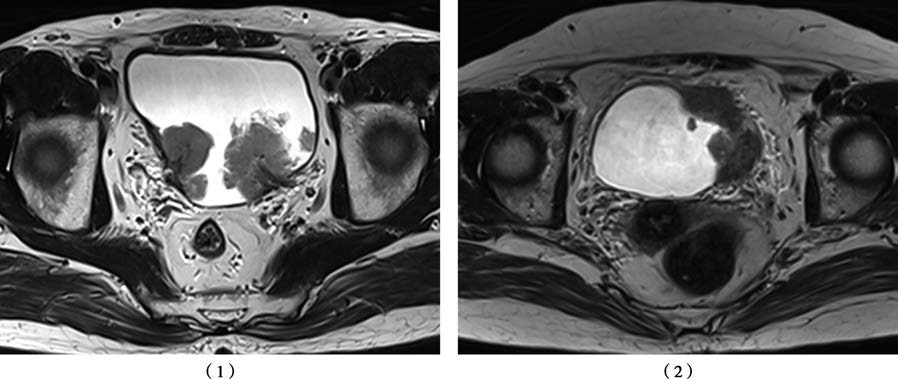
Figure 3 MRI characteristics of bladder cancer
(1) Bladder cancer, primary tumor staged as T1
(2) Bladder cancer, primary tumor staged as T3b
Cystoscopy and Biopsy
Pathological biopsy remains the most reliable method for diagnosing bladder cancer. Cystoscopy allows direct visualization of the tumor's location, size, number, and morphology (e.g., pedunculated or broad-based) and enables biopsy of the tumor or suspicious lesions.
In carcinoma in situ (Tis), localized mucosa may appear pale red and velvety, resembling congested mucosa. Low-grade papillary carcinomas typically appear as light red tumors with slender stalks and villiform branches. High-grade invasive carcinomas often present as dark red or brown nodular masses with a wide base, potentially accompanied by necrosis or calcification. Observations for tumor proximity to the ureteral orifices, bladder neck, and potential tumor growth within diverticula are crucial during the examination.
New techniques such as narrow-band imaging cystoscopy and blue-light cystoscopy help improve diagnostic accuracy for bladder cancer. When imaging studies clearly indicate tumor-like lesions in the bladder, diagnostic transurethral resection under anesthesia can provide a more accurate histological diagnosis, pathological grading, and clinical staging.
Treatment
A comprehensive treatment approach is adopted, with surgery being the primary modality.
Non-Muscle-Invasive Bladder Cancer (Tis, Ta, T1)
Transurethral resection of bladder tumor (TURBT) is commonly employed, followed by adjuvant intravesical chemotherapy or immunotherapy.
TURBT serves as both a critical diagnostic method and the first-line treatment for bladder cancer. The procedure involves complete removal of the tumor down to the normal bladder wall muscle layer using either electrocautery or laser techniques.
Although TURBT can completely remove Ta and T1 tumors, there remains a risk of recurrence or progression to muscle-invasive bladder cancer postoperatively. Therefore, adjuvant intravesical chemotherapy drugs or immunotherapeutic agents should be administered after surgery. Intravesical chemotherapy is typically initiated within 24 hours postoperatively. In intermediate- and high-risk patients, maintenance intravesical chemotherapy or immunotherapy is recommended. Commonly used chemotherapeutic agents include mitomycin C, epirubicin, and pirarubicin. Bacillus Calmette-Guérin (BCG) is considered the most effective agent for intravesical immunotherapy, with efficacy exceeding that of intravesical chemotherapy drugs, and is generally administered two weeks after surgery.
For high-risk non-muscle-invasive bladder cancer, including T1 high-grade tumors that are multifocal, recurrent, associated with Tis, unresponsive to BCG therapy, or non-urothelial bladder cancers, radical cystectomy is indicated.
Muscle-Invasive Bladder Cancer (T2–T4)
For resectable, non-metastatic muscle-invasive bladder cancer (T2–4aN0–xM0), the current standard treatment consists of neoadjuvant therapy combined with radical cystectomy and pelvic lymph node dissection, with postoperative adjuvant therapies such as chemotherapy, radiotherapy, or immunotherapy as needed.
The surgical resection scope includes the bladder, perivesical adipose tissue, the distal ureters, and pelvic lymph nodes. For male patients, the procedure typically involves removal of the prostate and seminal vesicles. For female patients, it includes removal of the uterus, adnexa, and anterior vaginal wall. In certain cases, a complete urethrectomy may also be necessary. Postoperative urinary diversion and reconstruction methods include orthotopic neobladder creation, ileal conduit, and cutaneous ureterostomy. Minimally invasive approaches, such as laparoscopic or robot-assisted laparoscopic radical cystectomy, have increasingly become the primary surgical techniques.
For patients unable or unwilling to undergo radical cystectomy, bladder-preserving multimodal therapies can be considered. After appropriate bladder-preserving surgery, adjuvant chemotherapy and radiotherapy, along with close follow-up, are recommended. If necessary, salvage cystectomy can be performed.
Chemotherapy is an essential adjunct to improve the efficacy of radical cystectomy and includes neoadjuvant chemotherapy before surgery and adjuvant chemotherapy afterward. Platinum-based combination chemotherapy regimens, such as GC (gemcitabine and cisplatin) and ddMVAC (dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin), are commonly used. Radiotherapy can be applied alone or in combination with chemotherapy.
For metastatic bladder cancer that cannot be cured surgically, systemic therapy is the primary treatment option. In cases of severe hematuria, urinary obstruction, or other symptoms, palliative cystectomy and urinary diversion may also be performed.
Recently, therapies such as antibody-drug conjugates, immune checkpoint inhibitors, and targeted therapies have shown promising results. These approaches are increasingly utilized in neoadjuvant or adjuvant settings, as well as for metastatic bladder cancer. Immunotherapy combined with chemotherapy or antibody-drug conjugates has demonstrated superior therapeutic outcomes.
Bladder Squamous Cell Carcinoma and Adenocarcinoma
Squamous cell carcinoma and adenocarcinoma are typically invasive bladder epithelial tumors with poor differentiation and high aggressiveness. At the time of confirmed diagnosis, these tumors are often in advanced stages. Radical cystectomy combined with pelvic lymph node dissection serves as the primary treatment approach.
Prognosis
The five-year overall survival rates for bladder cancer patients are approximately 90%–95% for stages Ta–T1, 69%–84% for stage T2, 35%–44% for stage T3, and 5%–10% for stage T4.