Benign prostatic hyperplasia (BPH) is the most common benign condition causing urinary obstruction in middle-aged and elderly men. It is primarily characterized by histological hyperplasia of the stromal and glandular components of the prostate, anatomical enlargement of the prostate, bladder outlet obstruction related to urodynamics, and lower urinary tract symptoms (LUTS) as the main clinical manifestation.
Epidemiology
The incidence of BPH increases with age. Histologically, BPH typically begins after the age of 40, with a prevalence of over 50% by the age of 60 and reaching as high as 83% by the age of 80. Similarly, the prevalence of LUTS rises with age, in parallel with histological changes. Approximately 50% of men with histological BPH experience moderate to severe LUTS. Reported clinical prevalence rates of BPH vary widely, likely due to differences in diagnostic criteria, study populations, and assessment methods. Among men over 50 years old, the clinical prevalence of BPH is between 50% and 75%, and this rate increases with advancing age, exceeding 80% in men over 70.
Etiology
Advancing age and functional testes are essential prerequisites for BPH. The incidence of BPH increases with age; after the age of 40, varying degrees of prostatic hyperplasia may develop, with clinical symptoms often emerging after 50. Additionally, the normal development of the prostate depends on androgens. Prepubertal castration prevents prostate development and precludes the occurrence of BPH later in life. In cases of BPH, castration leads to apoptosis of hyperplastic epithelial cells and subsequent glandular atrophy. However, the exact mechanisms underlying the development of BPH remain unclear, potentially involving disruptions in the balance between cell proliferation and apoptosis in epithelial and stromal cells. Contributing factors may include androgens and their interaction with estrogens, epithelial-stromal interactions within the prostate, growth factors, inflammatory cells, neurotransmitters, and genetic influences.
Pathology
Prostatic glandular hyperplasia begins in the glands surrounding the urethra, a region referred to as the transitional zone, which accounts for only 5% of the prostate prior to hyperplasia. The remaining prostate comprises the central zone (25%) and the peripheral zone (70%). The central zone is wedge-shaped and surrounds the ejaculatory ducts, while the peripheral zone constitutes the dorsal and lateral portions of the prostate and is the most common site of prostate cancer. BPH primarily affects the transitional zone around the prostatic urethra, where hyperplastic tissue forms multiple nodules that progressively enlarge. The hyperplastic glands compress and atrophy the surrounding peripheral glands, creating a distinct "surgical capsule" that separates the hyperplastic tissue from the peripheral zone and facilitates dissection during surgery.
Hyperplastic tissue protruding into the posterior urethra elongates, curves, and narrows the prostatic urethra, increasing urethral resistance and leading to difficulties in urination. The smooth muscle within the prostate, particularly around the bladder neck, contains numerous α-adrenergic receptors. Activation of these receptors causes smooth muscle contraction, further increasing resistance in the prostatic urethra.
BPH, combined with α-adrenergic receptor-mediated smooth muscle contraction in the posterior urethra, leads to bladder outlet obstruction. To counteract the increased resistance to urine outflow, the detrusor muscle compensates by enhancing its contractile force, gradually becoming hypertrophic and forming a coarse, trabeculated structure. Chronic high intravesical pressure results in the development of bladder trabeculae, diverticula, or pseudodiverticula. Progressive detrusor decompensation and reduced compliance may lead to detrusor instability, manifesting as significant urinary frequency, urgency, and urge incontinence.
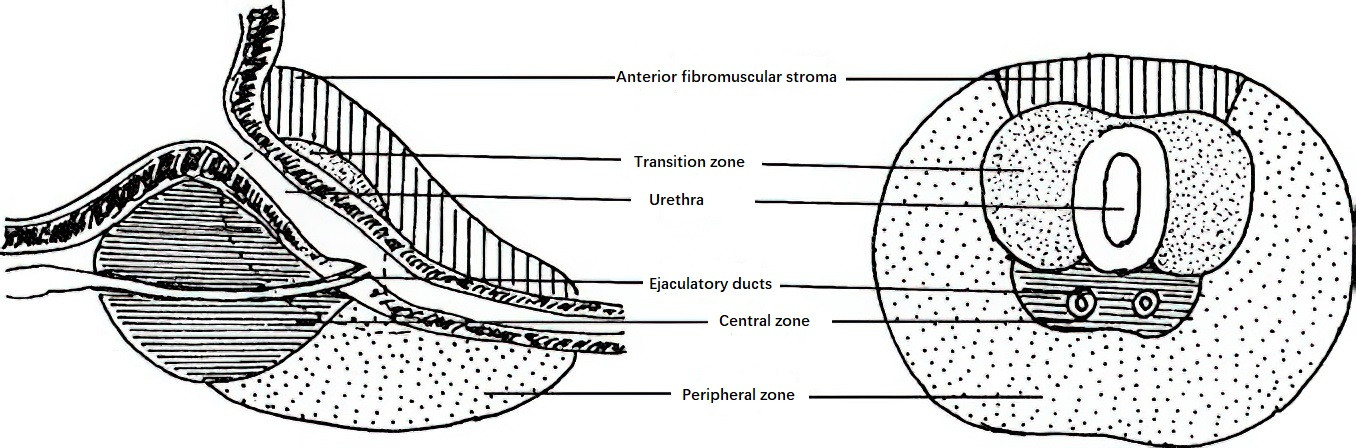
Figure 1 Normal anatomy of the prostate
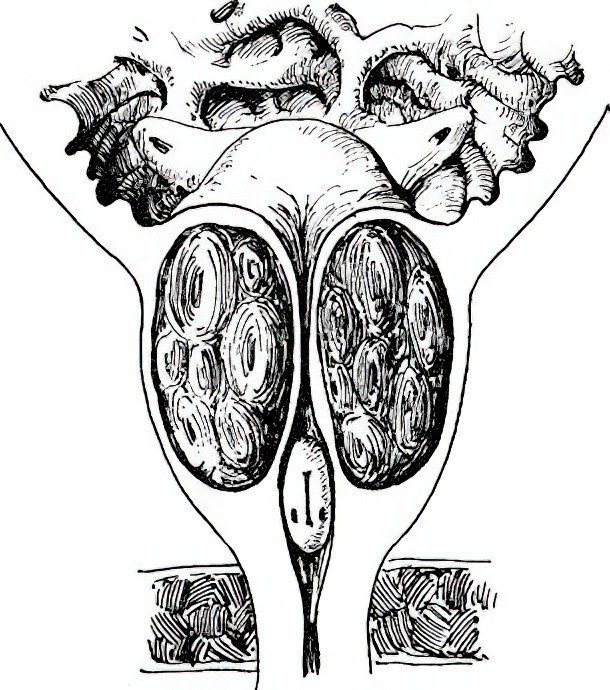
Figure 2 Changes in BPH
In BPH, the gland protrudes toward the posterior urethra and bladder neck, leading to elongation of the posterior urethra.
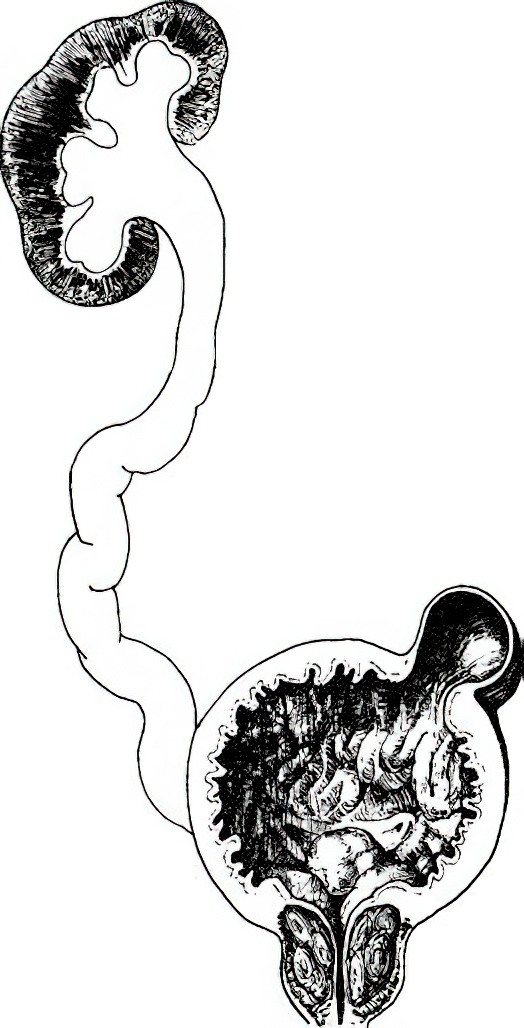
Figure 3 Pathological changes caused by BPH
Hydronephrosis: Renal parenchymal atrophy and renal pelvis dilation.
Ureteral Hydroureter: Ureteral dilation, elongation, and tortuosity.
Bladder Wall Hypertrophy: Thickening of the bladder wall, formation of muscular trabeculae, and bladder diverticula.
Bladder outlet obstruction may increase the resistance to urinary flow from the ureters, causing upper urinary tract dilation and hydronephrosis. If the obstruction persists, detrusor atrophy occurs, leading to weakened contractility and incomplete bladder emptying, resulting in residual urine. With increasing residual urine volume, the bladder wall may thin and the bladder cavity enlarge, eventually causing chronic urinary retention and overflow incontinence. Urinary reflux may further lead to upper urinary tract hydronephrosis and renal function impairment. Obstruction-induced urinary retention may also result in secondary infections and the formation of bladder stones.
Clinical Manifestations
Symptoms of benign prostatic hyperplasia (BPH) generally begin to appear after the age of 50 and become more pronounced around the age of 60. The severity of symptoms does not necessarily correlate with the size of the prostate but depends on the degree of obstruction, the rate of disease progression, and the presence of complications such as infection. Symptoms can fluctuate in intensity.
BPH primarily manifests as lower urinary tract symptoms (LUTS), which include storage symptoms, voiding symptoms, and post-voiding symptoms. Storage symptoms consist of urinary frequency, urgency, incontinence, and nocturia. Voiding symptoms include hesitancy, difficulty in urination, and intermittent flow. Post-voiding symptoms involve a sensation of incomplete emptying and post-micturition dribbling.
Urinary frequency is the most common early symptom of BPH, particularly noticeable at night. In the early stages, urinary frequency arises from prostatic hyperplasia and congestion that irritate the bladder. As the disease progresses and obstruction worsens, residual urine increases, reducing effective bladder capacity and further exacerbating urinary frequency. Obstruction may also lead to changes in detrusor muscle function, decreased bladder compliance, or detrusor instability, worsening urinary frequency and causing symptoms such as urgency incontinence.
Difficulty in urination is the most significant symptom of BPH, typically developing gradually. Classic signs include delayed initiation of urination, interrupted flow, weak and thin urinary stream, short urinary trajectory, terminal dribbling, and prolonged voiding time. In cases of severe obstruction and increased residual urine, patients often need to strain and increase abdominal pressure to aid urination, and they may experience a persistent sensation of incomplete emptying. When obstruction reaches a critical level, residual urine further increases, leading to chronic urinary retention or overflow incontinence. Various factors, including changes in weather, fatigue, alcohol consumption, constipation, or prolonged sitting, may suddenly worsen prostatic congestion and edema, resulting in acute urinary retention. This causes an inability to urinate, severe bladder distension, and intolerable lower abdominal pain, frequently requiring emergency catheterization.
When BPH is accompanied by infection or stones, obvious symptoms such as frequency, urgency, and painful urination may occur. Rupture of blood vessels on the surface of hyperplastic glandular mucosa can lead to varying degrees of painless gross hematuria, which must be distinguished from hematuria caused by urinary tract tumors. Severe obstruction with significant hydronephrosis or renal impairment may result in signs of chronic renal failure, such as loss of appetite, nausea, vomiting, anemia, and fatigue. Prolonged straining to urinate due to chronic difficulty in urination may also lead to complications such as inguinal hernia, internal hemorrhoids, or rectal prolapse.
Diagnosis
In men over 50 years of age presenting with LUTS and other related clinical manifestations, the possibility of BPH is considered. Clinical evaluation typically involves the following:
Medical History
Details of LUTS, including their characteristics, duration, and associated symptoms, are assessed. History of prior surgeries or trauma, particularly pelvic surgeries or injuries, is considered. Past medical history is evaluated for sexually transmitted diseases, diabetes, neurological disorders, or cardiac conditions potentially associated with nocturia. A medication history is also obtained to determine whether the patient has recently taken drugs that may affect urinary function or contribute to LUTS. General health, lifestyle habits, emotional and psychological factors are also explored.
Symptom Assessment
The International Prostate Symptom Score (IPSS) is the primary method for quantifying LUTS in men with BPH and is internationally recognized as the best tool for assessing BPH symptom severity.
Digital Rectal Examination (DRE)
This is an essential diagnostic method for BPH. Most patients with BPH exhibit an enlarged prostate with a smooth, firm, elastic surface and clear edges during DRE. The central groove is often shallower or absent. During the examination, attention is given to assessing anal sphincter tone and identifying any hard nodules in the prostate, which are critical for differentiating between neurogenic bladder and prostate cancer.
Ultrasound Examination
Ultrasound can be performed via the abdominal or transrectal approach. Abdominal ultrasound requires bladder filling and provides data on prostate volume (volume calculation formula: 0.52 × anteroposterior diameter × transverse diameter × longitudinal diameter), shape, and whether hyperplastic tissue is projecting into the bladder. It also evaluates for bladder stones or upper urinary tract hydronephrosis. Post-void residual urine volume can also be measured after the patient urinates. Transrectal ultrasound provides a clearer view of the internal structure of the prostate.
Uroflowmetry
Uroflowmetry is recommended when bladder urine volume is between 150–400 mL. A maximum urine flow rate (Qmax) of <15 mL/s indicates impaired urinary flow, while a Qmax of <10 mL/s suggests severe obstruction. For further evaluation of detrusor function and to determine if voiding difficulty is due to neurogenic bladder disorders, urodynamic studies may be performed.
Serum Prostate-Specific Antigen (PSA) Testing
Serum PSA testing is important for ruling out prostate cancer. However, multiple factors can influence PSA levels, including age, prostate volume, prostatitis, urinary tract infections, prostate massage, and transurethral procedures, all of which may lead to elevated PSA levels. For this reason, PSA testing is typically performed prior to DRE.
Additional tests such as intravenous urography (IVU), computed tomography (CT), magnetic resonance imaging (MRI), and cystoscopy may help identify concomitant conditions like urinary tract stones or tumors. Radionuclide renal scans are useful for assessing upper urinary tract obstruction and evaluating the degree of renal function impairment.
Differential Diagnosis
Urinary difficulty caused by benign prostatic hyperplasia (BPH) should be differentiated from the following conditions:
Prostate Cancer
If nodules are present in the prostate or the prostate feels hard on examination, or if serum PSA levels are elevated, magnetic resonance imaging (MRI) is indicated, and a prostate biopsy may be necessary for confirmation.
Bladder Neck Contracture
Also known as bladder neck fibrosis, this condition is often due to chronic inflammation, tuberculosis, or postoperative scar formation. It generally occurs in younger individuals, typically between the ages of 40 and 50, with symptoms of urinary obstruction, but without significant prostate enlargement. Diagnosis can be confirmed by cystoscopy.
Urethral Stricture
This condition is often associated with a history of urethral trauma or infection. Diagnosis can be confirmed through urethrocystography or urethroscopy.
Neurogenic Bladder
Although the clinical presentation resembles BPH, symptoms include difficulty urinating, increased residual urine, hydronephrosis, and impaired renal function. However, prostate enlargement is not observed, as the obstruction is functional. There is often a history of central or peripheral nervous system damage, along with clinical signs such as impaired lower limb sensation and movement, reduced perineal skin sensation, relaxation of the anal sphincter, or absence of reflexes. Intravenous urography typically reveals upper urinary tract dilation and hydronephrosis, while the bladder often exhibits a "Christmas tree" shape. Urodynamic studies can aid in the diagnosis.
Treatment
The principles of treatment include improving lower urinary tract symptoms, restoring normal urinary function, enhancing quality of life, slowing disease progression, and preventing complications. Treatment options for BPH are selected based on the patient’s symptoms, degree of obstruction, and presence of complications.
Watchful Waiting
For patients with mild symptoms that do not affect quality of life and are not associated with complications, treatment may not be necessary initially, and observation may be considered. Regular follow-up is advised, as symptoms may worsen with increasing prostate volume and age, eventually requiring treatment.
Medication Therapy
Commonly used medications include α-adrenergic receptor antagonists (α-blockers) and 5α-reductase inhibitors. Other options include muscarinic receptor antagonists, phosphodiesterase type-5 inhibitors, β3-adrenergic agonists, and herbal preparations. Combination therapy may be used in certain patients depending on their clinical condition.
α-adrenergic receptors are classified into α1 and α2· subtypes, with α1 receptors predominantly located in the smooth muscle of the prostatic stroma. These receptors significantly influence voiding. Blocking α1 receptors effectively reduces smooth muscle tension at the bladder neck and in the prostate, decreasing urethral resistance and improving urinary function. Common agents include terazosin, alfuzosin, doxazosin, and tamsulosin, which show good efficacy in patients with mild symptoms and smaller prostate volumes. These medications take effect quickly, with symptom improvement typically observed within hours to days. They can reduce the International Prostate Symptom Score (IPSS) by approximately 30-40% on average and improve maximum urine flow rate by 16-25%. Side effects are generally mild and may include dizziness, nasal congestion, and orthostatic hypotension.
5α-reductase inhibitors block the conversion of testosterone to the active dihydrotestosterone within the prostate, resulting in partial reduction of prostate volume and improvement in urinary symptoms. Commonly used drugs include finasteride, which takes effect more slowly, typically within three months of therapy. Symptoms often relapse upon discontinuation, necessitating long-term use. Finasteride can reduce prostate volume by 20-30%, decrease IPSS by an average of 15%, and increase maximum urine flow rate by 1.3-1.6 mL/s. It is particularly effective in patients with larger prostates and shows enhanced efficacy when combined with α-blockers.
Surgical Treatment
Surgical treatment is considered for patients with moderate to severe LUTS that significantly affect quality of life, especially when medical therapy is ineffective or declined. Surgical intervention is recommended when BPH leads to the following complications:
- Recurrent urinary retention;
- Recurrent hematuria;
- Recurrent urinary tract infections;
- Bladder stones;
- Secondary upper urinary tract hydronephrosis;
- Complications such as inguinal hernia, severe hemorrhoids, or rectal prolapse, where the clinical assessment suggests that resolution of lower urinary tract obstruction is necessary to achieve therapeutic success.
The choice of surgical method depends on prostate volume, institutional resources, the surgeon's expertise, the patient’s preference, and the presence of coexisting diseases or overall health status.
Open Prostatectomy (OP)
This is the most conventional surgical method for BPH. It typically involves a suprapubic or retropubic approach, with the hyperplastic glandular tissue enucleated along the surgical capsule under finger guidance. Although this procedure provides definitive outcomes, it is associated with significant trauma due to the prostate’s deep location and vascular supply, resulting in more postoperative complications and slower recovery. Open prostatectomy is now rarely performed, except in certain situations, such as in hospitals without transurethral prostate surgery capability or in cases of extremely large prostates (over 80 mL) combined with large bladder stones.
Transurethral Resection or Incision of the Prostate
Transurethral Resection of the Prostate (TURP)
TURP is currently considered the standard surgical procedure for BPH and is primarily indicated for patients with a prostate volume of less than 80 mL. The principle of TURP involves resecting hyperplastic prostatic tissue from the urethral surface of the prostate toward the surgical capsule. Based on the energy platform used, TURP can be categorized into monopolar TURP (M-TURP), bipolar TURP (B-TURP), and TURP performed with various wavelengths of lasers.
Transurethral Incision of the Prostate (TUIP)
TUIP involves creating one or two longitudinal grooves extending to the surgical capsule at the 5–7 o’clock positions of the prostate, without completely removing the hyperplastic prostatic tissue surrounding the urethra. This procedure is mainly indicated for patients with a prostate volume under 30 mL who do not have median lobe hyperplasia. The efficacy of TUIP is comparable to TURP, but it offers greater safety.
Transurethral Enucleation of the Prostate
This technique combines the principles of open prostatectomy with the minimally invasive benefits of TURP, resulting in endoscopic enucleation of the prostate (EEP). The technical approach involves using different energy platforms (electrosurgical or laser) under endoscopic guidance to completely enucleate the hyperplastic tissue along the surgical capsule, followed by removal of the enucleated tissue using cutting or tissue morcellation techniques to relieve obstruction. Due to its superior long-term efficacy and safety, EEP is increasingly utilized and is considered the preferred surgical option for patients with large prostates (over 80 mL). Commonly used energy platforms include:
- Electrosurgery: Plasma electrodes.
- Laser: Holmium laser, thulium laser, green laser, and various semiconductor lasers with different wavelengths, such as a 450-nm blue laser, a 980-nm red laser, and a 1,470-nm laser.
Transurethral Vaporization of the Prostate
This procedure is based on principles similar to those of TURP and primarily utilizes energy platforms such as lasers to vaporize hyperplastic prostatic tissue from the urethral surface toward the surgical capsule. It is indicated for patients with prostate volumes less than 80 mL.
Other Treatments
Procedures such as transurethral balloon dilation, prostatic water vapor therapy, prostatic artery embolization, prostatic urethral stenting, and transrectal high-intensity focused ultrasound (HIFU) have shown some efficacy in alleviating BPH-related obstructive symptoms. These approaches are suitable for patients who are unable to tolerate surgery or have specific treatment preferences.
Follow-up
All patients undergoing treatment for BPH require follow-up. The purpose of follow-up is to assess disease progression, treatment efficacy, and any related side effects or complications, as well as to propose further management strategies.
Watchful Waiting
Patients under observation require periodic follow-up. The first follow-up visit is typically scheduled six months after the initial assessment, followed by annual follow-ups. If symptoms worsen or surgical indications become evident, treatment plans should be adjusted promptly. Follow-up evaluations typically include IPSS, quality-of-life scores, urine analysis, uroflowmetry, and measurement of post-void residual volume. When necessary, annual digital rectal examinations and serum PSA testing are also performed.
Medication Therapy
The timing of follow-up visits for patients on medication is determined by the effectiveness of the drugs, their side effects, the convenience of medical consultations, and the treating physician's experience. In addition to the assessments mentioned above, particular attention is given to evaluating the efficacy of the medications and monitoring for side effects. This may involve testing for serum creatinine levels and performing ultrasound examinations of the urinary system and prostate. Follow-up results are used to adjust the treatment regimen accordingly.
Surgical Therapy
The first follow-up after surgery is usually scheduled 4–6 weeks after the urinary catheter is removed. In addition to the tests mentioned above, the initial postoperative follow-up includes assessments for residual urine, new lower urinary tract symptoms, urinary incontinence, or gross hematuria. If warranted, diagnostic procedures such as digital rectal examination and serum PSA testing are also conducted.