Portal hypertension refers to a clinical syndrome caused by elevated pressure in the portal venous system due to various factors that obstruct portal blood flow and/or increase the volume of blood flowing through the portal system. This leads to complications such as splenomegaly, hypersplenism, esophageal and gastric varices, hematemesis or melena, and ascites. Normal portal pressure ranges from 13 to 24 cmH2O, with an average value of 18 cmH2O, which is typically 5–9 cmH2O higher than hepatic venous pressure. Portal hypertension is defined as portal pressure exceeding 25 cmH2O, with most cases reaching pressures of 30–50 cmH2O.
Anatomical Overview
The portal venous system is distinct from the systemic venous system in two primary ways: it lies between two capillary networks, and it lacks valves within the vascular system. The main trunk of the portal vein is formed by the convergence of the superior mesenteric vein, inferior mesenteric vein, and splenic vein. These veins originate from capillary networks in the stomach, intestines, spleen, and pancreas, which gradually merge to form the main portal vein. Near the hepatic hilum, the main portal vein divides into left and right branches, which enter the left and right hepatic lobes, respectively, and further divide into smaller branches. These smaller branches ultimately merge with the small branches of the hepatic artery within the liver lobules, converging into hepatic sinusoids (the liver’s capillary network). Blood from the sinusoids flows into the central veins of liver lobules, then drains into sublobular veins, hepatic veins, and eventually into the inferior vena cava.
In addition to converging within the hepatic sinusoids, the small branches of the portal vein and hepatic artery are interconnected via numerous small arteriovenous communications located in the interlobular portal tracts. These arteriovenous communications generally remain closed under normal conditions but open when hepatic blood flow increases. The total hepatic blood flow in a healthy individual is approximately 1,500 mL per minute, of which about 1,125 mL (60%–80%, with an average of 75%) is attributable to the portal vein and about 375 mL (20%–40%, with an average of 25%) to the hepatic artery. Despite the portal vein supplying the majority of hepatic blood flow, the hepatic artery, with its higher pressure and oxygen content, contributes equally to the liver's oxygen supply.
There are four collateral pathways between the portal venous system and the systemic venous system. Under normal circumstances, these pathways are narrow, with minimal blood flow.
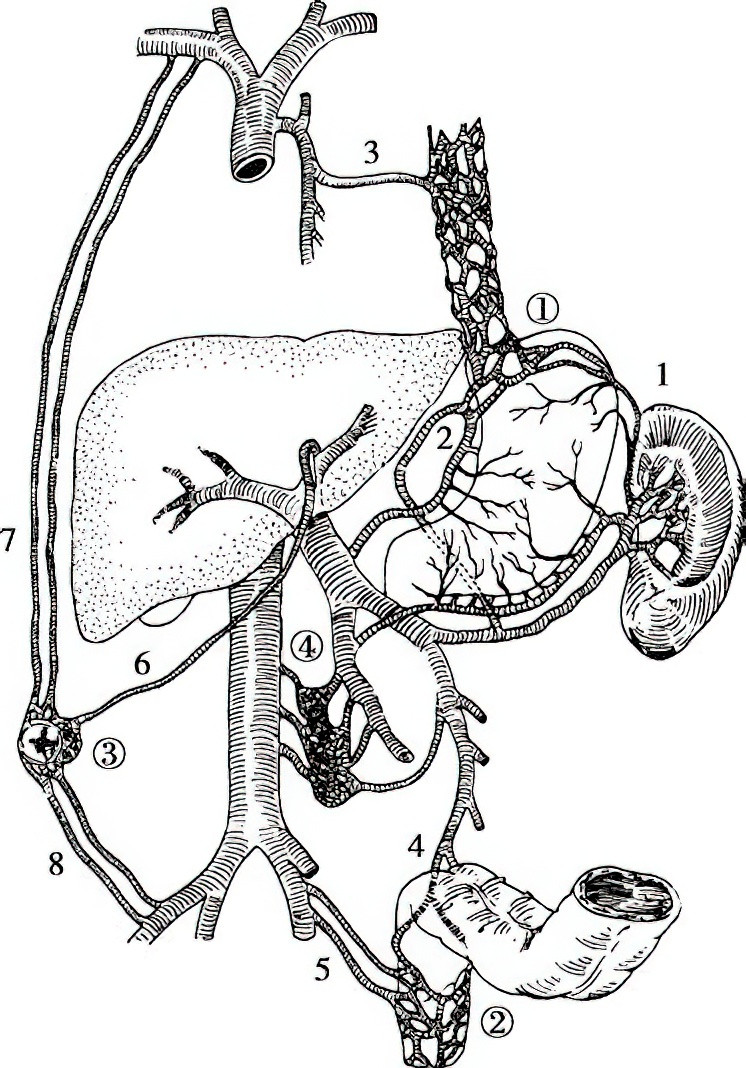
Figure 1 Collateral pathways between the portal and systemic veins
1, Short gastric vein
2, Left gastric (coronary) vein
3, Azygos vein
4, Superior rectal vein
5, Inferior rectal vein and anal vein
6, Paraumbilical veins
7, Superior epigastric vein
8, Inferior epigastric vein
① Gastroesophageal collateral pathway
② Rectoanal collateral pathway
③ Anterior abdominal wall collateral pathway
④ Retroperitoneal collateral pathway
Gastric Fundus and Lower Esophagus Collateral Pathway
Blood from the portal vein flows through the left gastric vein and short gastric veins, forming anastomoses with branches of the azygos and hemiazygos veins via the esophageal-gastric venous plexus, eventually draining into the superior vena cava.
Lower Rectum and Anal Canal Collateral Pathway
Blood from the portal vein flows through the inferior mesenteric vein and the superior rectal vein, anastomosing with the inferior rectal and anal veins, and drains into the inferior vena cava.
Anterior Abdominal Wall Collateral Pathway
Blood from the left branch of the portal vein flows through the paraumbilical veins, which anastomose with the deep superior and inferior epigastric veins, ultimately draining into the superior and inferior vena cava, respectively.
Retroperitoneal Collateral Pathway
Branches of the superior and inferior mesenteric veins form anastomoses with branches of the inferior vena cava within the retroperitoneum, creating a collateral network known as the Retzius venous plexus.
Pathophysiology
The pressure in the portal vein is determined and sustained by the balance between inflowing blood volume and outflow resistance. Increased portal venous resistance is a common initiating factor in portal hypertension. Based on the site of resistance, portal hypertension is categorized into prehepatic, intrahepatic, and posthepatic types.
Prehepatic Portal Hypertension
Common causes include extrahepatic portal vein thrombosis (due to omphalitis, abdominal infections such as acute appendicitis and pancreatitis, trauma, etc.), congenital abnormalities (such as atresia, stenosis, or cavernous transformation), and external compression (such as from metastatic carcinoma or pancreatitis). Patients with extrahepatic portal vein obstruction often have normal or only mildly impaired liver function, with a better prognosis compared to intrahepatic portal hypertension.
Intrahepatic Portal Hypertension
This type is further classified into presinusoidal, sinusoidal, and postsinusoidal types. Presinusoidal obstructive portal hypertension is commonly caused by schistosomiasis. Hepatitis and liver cirrhosis are frequent causes of sinusoidal and postsinusoidal obstructive portal hypertension. In hepatic cirrhosis, proliferating fibrous septa and regenerating hepatic nodules compress and narrow or obliterate the hepatic sinusoids within lobules, obstructing portal blood flow and thereby increasing portal pressure. Furthermore, numerous arteriovenous communications located in the portal tracts between small branches of the hepatic artery and portal vein, normally closed, open significantly when hepatic sinusoids are compressed and obstructed. These communications allow high-pressure hepatic arterial blood—approximately 8–10 times the pressure of portal vein blood—to flow directly into lower-pressure portal vein branches, further elevating portal pressure.
Posthepatic Portal Hypertension
Common causes include Budd-Chiari syndrome, constrictive pericarditis, and severe right-heart failure.
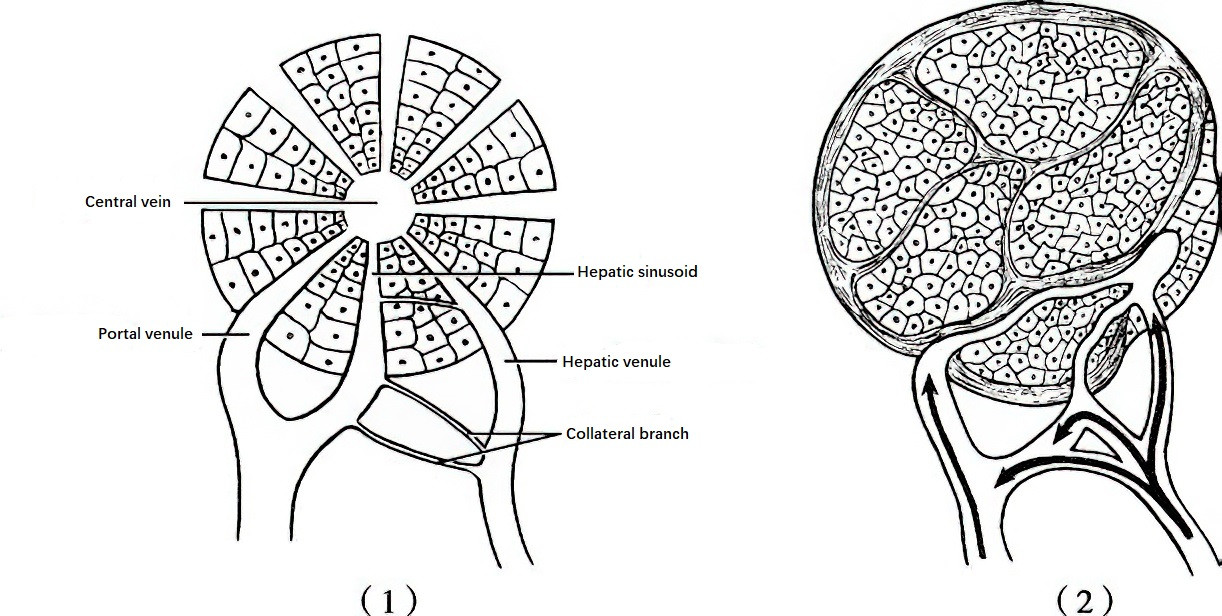
Figure 2 The Role of collateral pathways between portal and hepatic arterial branches in portal hypertension
1, Under normal conditions, portal vein and hepatic arterial branches flow into the hepatic sinusoids, and their collateral pathways are thin and closed.
2, In cirrhosis, the collateral pathways open, allowing high-pressure arterial blood to flow into the low-pressure portal vein, further exacerbating portal hypertension.
Persistent portal hypertension due to these conditions may result in the following pathological changes:
Splenomegaly and Hypersplenism
Elevated portal pressure leads to impaired splenic venous outflow, causing splenic sinusoidal dilation, splenic pulp hyperplasia, and splenomegaly. Increased splenic blood transit time enhances the likelihood of erythrocyte, leukocyte, and platelet destruction by splenic macrophages. Enhanced phagocytic activity of splenic macrophages leads to excessive destruction of blood cells, resulting in peripheral cytopenia, manifesting as leukopenia, thrombocytopenia, and anemia, which is collectively referred to as hypersplenism.
Dilatation of Collateral Pathways
When the normal intrahepatic portal vein pathways are obstructed, the four collateral pathways mentioned earlier open excessively and undergo dilation, distortion, and varicosity. The most clinically significant varices form in the lower esophagus and gastric fundus, as they are located closest to the main portal vein and systemic venous drainage and experience the largest pressure gradient. Consequently, these varices are affected earliest and most significantly by portal hypertension. Patients with cirrhosis frequently experience gastric acid reflux, leading to reflux esophagitis, which compromises the integrity of the lower esophageal mucosa. Mechanical injuries caused by hard or rough foods, as well as increased intra-abdominal pressure due to coughing, vomiting, straining during defecation, or lifting heavy objects, can cause varices to rupture, resulting in life-threatening hemorrhage. Other collateral pathways may also undergo dilation, such as the superior and inferior rectal venous plexuses, which can lead to secondary hemorrhoids. Dilated paraumbilical veins and their connections to the superior and inferior epigastric veins can cause anterior abdominal wall varices, sometimes forming the characteristic "caput medusae." Retroperitoneal collateral veins have less clinical significance but, in rare cases, may rupture and cause retroperitoneal hematomas.
Ascites
Elevated portal vein pressure increases the filtration pressure in the capillary bed of the portal system. Concurrently, cirrhosis-induced hypoalbuminemia decreases plasma colloid osmotic pressure, and enhanced lymph production contributes to fluid leakage into the peritoneal cavity, resulting in ascites. Portal hypertension increases portal venous blood flow, reducing effective circulating volume, which stimulates excessive aldosterone secretion. Additionally, impaired hepatic inactivation of aldosterone and antidiuretic hormone during chronic liver disease exacerbates sodium and water retention, further promoting ascites formation.
Portal hypertension also results in gastric wall congestion and edema, with widespread opening of submucosal arteriovenous communications in the stomach. These microcirculatory abnormalities can compromise the gastric mucosal barrier, leading to portal hypertensive gastropathy, which occurs in approximately 20% of cases and accounts for 5%–20% of portal hypertension-related upper gastrointestinal bleeding.
Moreover, in portal hypertension, large volumes of portal venous blood bypass hepatocytes via natural or surgical portosystemic shunts. Coupled with severe hepatic parenchymal dysfunction, toxic substances such as ammonia, thiols, and γ-aminobutyric acid enter the systemic circulation, leading to neurotoxic effects on the brain and manifesting as neuropsychiatric syndrome. This condition is referred to as hepatic encephalopathy and is frequently triggered by gastrointestinal bleeding, infections, excessive protein intake, or the use of sedatives and diuretics.
Clinical Manifestations
The primary manifestations include splenomegaly and hypersplenism, hematemesis or melena, ascites, and nonspecific systemic symptoms, most of which are related to impaired liver function. These systemic signs include fatigue, drowsiness, anorexia, facies characteristic of chronic liver disease, spider nevi, palmar erythema, gynecomastia, and testicular atrophy. Rupture of esophageal or gastric varices leads to acute, massive hemorrhage, with the patient vomiting bright red blood. Coagulopathy caused by liver dysfunction and thrombocytopenia caused by hypersplenism further impair the ability to achieve hemostasis. Severe hypoxia in hepatic tissue due to massive hemorrhage can predispose patients to hepatic encephalopathy.
On physical examination, a palpable spleen strongly suggests the presence of portal hypertension. Signs such as jaundice, ascites, and dilated veins over the anterior abdominal wall indicate severe portal hypertension. Early-stage liver disease may present with a liver that feels firm, has blunted and irregular edges, and is palpable; however, in most cases, advanced liver cirrhosis causes liver shrinkage, making it difficult to palpate.
Auxiliary Examinations
Complete Blood Count
Hypersplenism often leads to reduced blood cell counts, with leukocyte counts below 3 × 109/L and platelet counts below (70–80) × 109/L being the most common findings. Anemia may result from bleeding, malnutrition, hemolysis, or bone marrow suppression.
Liver Function Tests
Liver function tests usually show decreased plasma albumin levels and elevated globulin levels, with a reversed albumin-to-globulin (A/G) ratio. Because many coagulation factors are synthesized in the liver, and chronic liver disease is often accompanied by primary hyperfibrinolysis, prolonged prothrombin time is frequently observed. Testing for hepatitis markers and alpha-fetoprotein is also necessary. Liver function grading, liver volume measurement through CT scanning, and indocyanine green (ICG) clearance testing can provide important information for evaluating hepatic reserve function and guiding clinical decisions.
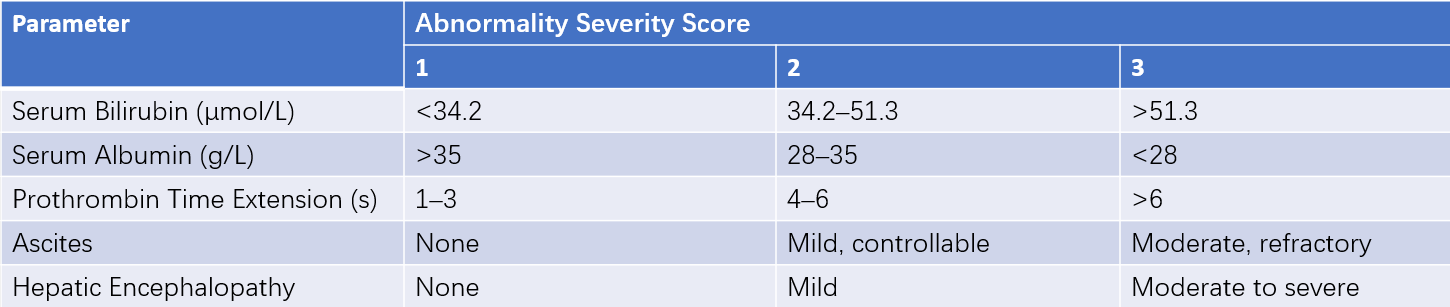
Table 1 Child-Pugh classification
Note: A total score of 5–6 indicates good liver function (Class A). A score of 7–9 indicates moderate liver function (Class B), and a score of 10 or higher indicates poor liver function (Class C).
Abdominal Ultrasound
Ultrasound imaging can reveal ascites, abnormal liver density and texture, portal vein dilatation, vascular patency, and the diameters, flow velocities, and flow volumes of the portal vein, splenic vein, and hepatic artery. The direction of blood flow in the main portal vein and the presence of thrombosis in the portal venous system can also be assessed. In portal hypertension, the portal vein diameter is typically ≥1.3 cm, and the splenic vein diameter is often ≥0.8 cm.
Bone Marrow Examination
Bone marrow examination helps exclude splenomegaly caused by extramedullary hematopoiesis in patients with bone marrow fibrosis, preventing unnecessary splenectomy. It also evaluates the recovery of peripheral blood cells after splenectomy.
Barium Esophagogram and Endoscopy
In the esophagus, varices are seen as an eroded or moth-eaten appearance when barium fills the esophagus, and as worm-like or beaded filling defects when the esophagus is empty. Barium studies can also reveal gastric fundus varices or differentiate ulcers in the stomach and duodenum. However, these findings can be more directly observed through endoscopy. Endoscopy offers a detailed assessment of variceal severity, extent, and the presence of "red signs"; it also evaluates portal hypertensive gastropathy, assesses bleeding risk, and identifies the cause of upper gastrointestinal bleeding, making it more commonly used in clinical practice.
CT, CT Angiography (CTA), or Magnetic Resonance Portal Venography (MRPVG)
These imaging modalities help assess the degree of liver cirrhosis, the size and volume of the liver and spleen, and the presence and extent of portal venous thrombosis. They also provide measurements of the diameters of the hepatic and splenic arteries, the portal vein, and the splenic vein, as well as information about hepatic blood flow and the presence of hepatic tumors. Additionally, the location, size, and extent of collateral veins can be identified, which is helpful for surgical planning. It is important to avoid dilated abdominal wall veins when determining surgical incision sites or puncture points to preserve natural shunt pathways whenever possible.
Wedged Hepatic Venous Pressure (WHVP) and Hepatic Venous Pressure Gradient (HVPG)
These can be measured by inserting a balloon catheter into the hepatic vein through the internal jugular or femoral vein. The WHVP reflects sinusoidal pressure and indirectly estimates portal venous pressure in sinusoidal causes of portal hypertension. HVPG is calculated as the difference between WHVP and free hepatic venous pressure (FHVP), reflecting the pressure gradient between the portal vein and the intra-abdominal inferior vena cava. Unlike WHVP, HVPG eliminates the influence of intra-abdominal pressure on the results, providing a more accurate representation of portal venous pressure. Normal HVPG ranges from 3–5 mmHg, and values above 5 mmHg confirm the diagnosis of portal hypertension. Values exceeding 12 mmHg are associated with a high risk of variceal bleeding. However, because this is an invasive procedure, its clinical use is limited.
Diagnosis and Differential Diagnosis
The diagnosis relies primarily on the clinical history of liver diseases, such as hepatitis, autoimmune hepatitis, and schistosomiasis, in conjunction with clinical manifestations including splenomegaly, hypersplenism, hematemesis or melena, and ascites, as well as findings from auxiliary tests. Diagnosis is typically straightforward. During episodes of acute massive bleeding, differentiation from other causes of bleeding is necessary. Splenomegaly may also require differentiation from splenomegaly caused by hematologic disorders.
Treatment
Treatment primarily focuses on managing esophagogastric variceal rupture bleeding, splenomegaly with hypersplenism, refractory ascites, and underlying liver disease.
Esophagogastric Variceal Rupture Bleeding
Non-surgical Treatment
Non-surgical approaches are suitable for patients in poor general condition, those with significant liver dysfunction who cannot tolerate surgery, or as a preoperative preparation.
Fluid Resuscitation and Blood Transfusion
During acute bleeding, establishing effective intravenous access to administer fluids is essential. The patient's vital signs are closely monitored. For severe bleeding or hemoglobin levels below 70 g/L, blood transfusion is performed to restore effective blood volume. Once hemodynamic stability is achieved and hemoglobin is maintained at approximately 80 g/L, the transfusion and fluid resuscitation rate can be slowed to avoid excessive replenishment, which could trigger a rebound increase in portal pressure and lead to rebleeding.
Medications
Hemostasis
Vasoconstrictors are the first-line treatment during acute bleeding. Terlipressin (glypressin), administered intravenously at an initial dose of 2 mg (injection time >1 minute), is followed by 2 mg every 4 hours. If bleeding is controlled, the dosage can be reduced to 1 mg every 4 hours. Somatostatin or its octapeptide derivative, octreotide, are also frequently used. Somatostatin is given as an initial intravenous injection of 250 μg, followed by continuous intravenous infusion at 250–500 μg/h. Octreotide is administered as an initial intravenous injection of 50 μg, followed by continuous infusion at 25–50 μg/h. Both drugs are recommended for 3–5 days. Unlike other vasoconstrictors, somatostatin and octreotide do not cause systemic hemodynamic changes and have minimal short-term adverse reactions, making them widely used for esophagogastric variceal bleeding. However, early rebleeding frequency with medication alone remains high, necessitating additional measures to prevent recurrence. Long-term use of beta-blockers such as propranolol or carvedilol can help prevent rebleeding.
Infection Prevention
Broad-spectrum cephalosporin antibiotics are used to prevent infection.
Other Supportive Treatments
These may include proton pump inhibitors to reduce gastric acid secretion, diuretics, hepatic encephalopathy prevention, and comprehensive hepatoprotective treatments.
Endoscopic Treatment
If bleeding cannot be controlled within 24 hours of intensive medication therapy, endoscopic interventions are indicated. Two methods are commonly used:
Endoscopic Injection Sclerotherapy (EIS)
Sclerosants (e.g., polidocanol) are injected directly into or adjacent to varices through endoscopy. This method induces variceal thrombosis and obliteration, which is effective for treating gastric variceal bleeding and preventing rebleeding. Complications may include esophageal ulceration, stricture, or perforation. Although esophageal perforation is rare (occurs in 1% of cases), its mortality rate can reach 50%.
Endoscopic Variceal Ligation (EVL)
This technique involves endoscopic suction of the varices into a ligation device, followed by application of rubber bands at the base of the varices to achieve hemostasis. EVL is simpler and safer compared to sclerotherapy and is recognized as the first-line choice for controlling acute bleeding. Combined use with medication therapy achieves a treatment success rate of 80%–100%. Both EIS and EVL usually require multiple sessions at intervals of 2–4 weeks.
Balloon Tamponade with a Sengstaken-Blakemore Tube
Balloon tamponade can be employed when endoscopic therapy is unavailable or has failed. The tube consists of three lumens: one inflates the gastric balloon to compress the gastric fundus, another inflates the esophageal balloon to compress the lower esophageal varices, and the third allows for suction, irrigation, and administration of hemostatic agents. The inflated balloons exert pressure on the varices located in the gastric fundus and distal esophagus to achieve temporary hemostasis. This technique is effective for emergency bleeding control.
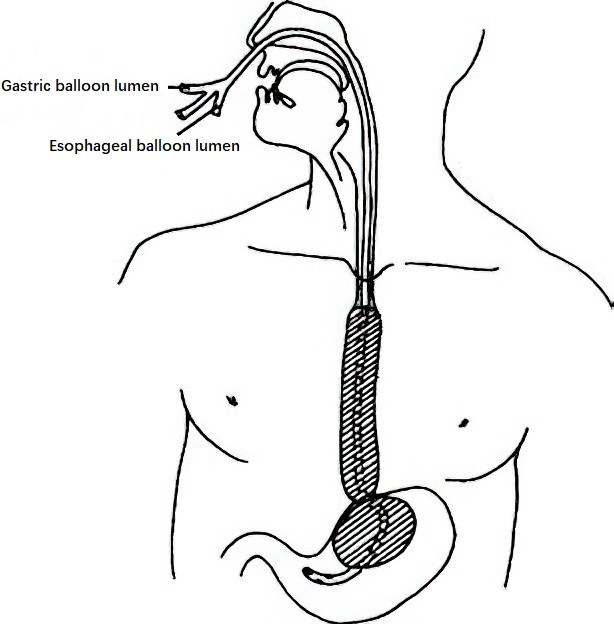
Figure 3 Three-lumen Sengstaken-Blakemore tube for bleeding control
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
TIPS is a radiological intervention where a channel is created between the hepatic vein and a major portal vein branch through the liver parenchyma, followed by placement of a stent to establish portal-systemic shunting. The stent diameter typically ranges from 8 to 12 mm. TIPS significantly reduces portal pressure and is indicated for acute bleeding control, prevention of rebleeding, and in patients awaiting liver transplantation. However, TIPS is associated with complications such as liver failure (5%–10% incidence) and hepatic encephalopathy (20%–40% incidence). Stent thrombosis and stenosis over time can reduce shunt efficacy, but the use of covered stents can lower the thrombosis rate.
####Surgical Treatment
Surgical treatment is considered for patients with a history or current episode of gastrointestinal bleeding, significant varices with "red signs" that indicate a high risk of bleeding, and those in generally good condition with relatively preserved liver function (Child-Pugh class A or B) who are estimated to tolerate surgery well. Patients classified as Child-Pugh class C are generally not recommended for surgery and are instead managed with non-surgical approaches.
Time to Surgery
The time to surgery is categorized into emergency surgery, elective surgery, and prophylactic surgery.
Emergency Surgery
Emergency surgery is performed for patients with massive and aggressive bleeding that cannot be controlled within 48 hours of intensive medical treatment or for those who experience rebleeding within 24 hours after achieving temporary hemostasis. However, these patients often present in critical condition with complications such as shock, resulting in high perioperative mortality rates.
Elective Surgery
For patients with a history of bleeding, elective surgery performed after adequate preoperative preparation can prevent rebleeding and reduce the occurrence of hepatic encephalopathy.
Prophylactic Surgery
For patients without a history of bleeding, prophylactic surgery is not generally recommended unless they present with significant splenomegaly and hypersplenism. In such cases, surgery can help alleviate hypersplenism while supporting the management of liver disease. Prophylactic surgery is also advised for patients with severe varices in the esophagogastric region, especially if "red signs" are observed during endoscopy.
Choice of Surgical Methods
The surgical methods for portal hypertension are broadly divided into shunt surgery, devascularization procedures, combination surgeries, and liver transplantation.
Shunt Surgery
Shunt surgery involves creating a pathway between the portal venous system and the systemic venous system to reduce portal pressure and achieve hemostasis.
Advantages are effective pressure reduction with a low rate of rebleeding.
Disadvantages are reduced portal venous blood flow to the liver, which may worsen liver function, and a high incidence of hepatic encephalopathy. Shunt surgery is not suitable for patients with advanced liver dysfunction. It is more appropriate for patients with a history of variceal bleeding, significant portal hypertensive gastropathy, or recurrent bleeding after prior devascularization procedures. Shunt surgeries are categorized into nonselective and selective shunts (including restrictive shunts).
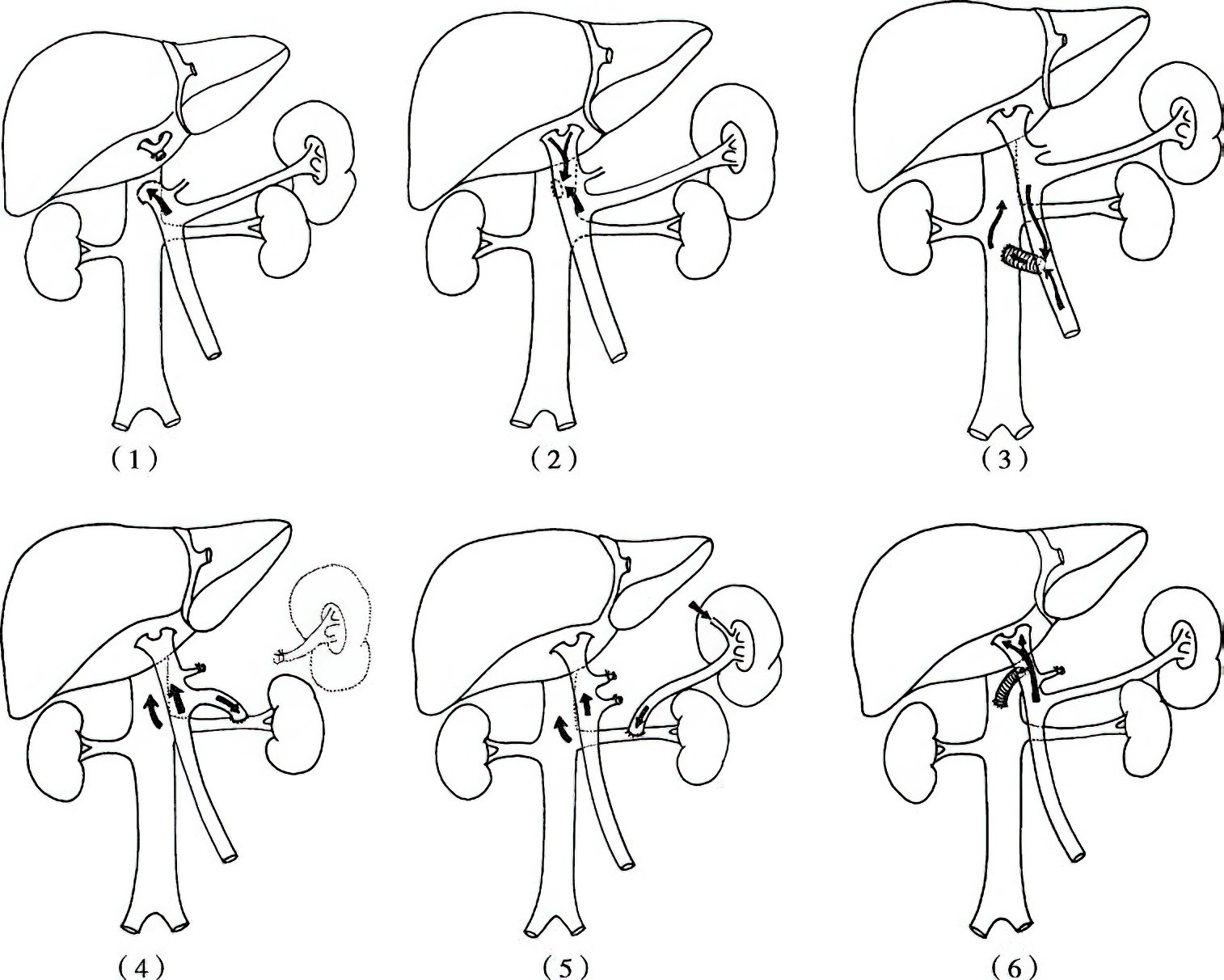
Figure 4 Shunt procedures
1, End-to-side portocaval shunt
2, Side-to-side portocaval shunt
3, Mesocaval ("H-shaped") shunt between superior mesenteric vein and inferior vena cava
4, Proximal splenorenal shunt
5, Distal splenorenal shunt
6, Restrictive portocaval "H-shaped" shunt
A. Nonselective Portosystemic Shunts
These shunts divert all portal venous blood flow into the systemic circulation. Representative procedures include:
- End-to-side portocaval shunt: Involves ligation of the hepatic end of the portal vein to prevent retrograde flow of blood into the liver.
- Side-to-side portocaval shunt: Redirects portal venous inflow into the inferior vena cava, reducing hepatic sinusoidal pressure and mitigating ascites formation.
- Mesocaval shunt ("H-shaped") using the superior mesenteric vein and the inferior vena cava.
- Proximal splenorenal shunt: Involves splenectomy and end-to-side anastomosis of the proximal splenic vein to the left renal vein.
Nonselective shunts effectively treat esophagogastric variceal bleeding but are associated with hepatic encephalopathy rates of 30%–50% and an increased risk of liver failure. Additionally, nonselective shunts often disrupt the anatomy of the porta hepatis, complicating any potential future liver transplantation.
B. Selective Portosystemic Shunts
Selective shunts aim to preserve portal venous inflow to the liver while reducing pressure in the esophagogastric varices.
Distal splenorenal shunt (DSRS) involves end-to-side anastomosis of the distal splenic vein to the left renal vein, along with the division of portal-systemic collateral channels, including the coronary vein and gastroepiploic veins. DSRS has a lower rate of hepatic encephalopathy compared to nonselective shunts. However, it is generally avoided in patients with severe ascites or a small-caliber splenic vein.
Restrictive shunts aim to sufficiently decrease portal pressure to prevent variceal bleeding while maintaining partial portal venous inflow to the liver.
Representative procedures include restrictive portocaval side-to-side shunts with a controlled anastomotic diameter (10 mm) and "H-shaped" mesocaval shunts using artificial grafts with diameters of 8–10 mm. While the former may gradually expand into a nonselective shunt over time, the latter carries a risk of thrombosis in the short term, necessitating thrombolytic or thrombectomy interventions.
Devascularization Procedures
Devascularization procedures involve interrupting the abnormal portal-systemic collateral circulation to achieve hemostasis.
Advantages include simple surgical techniques, minimal trauma, preservation of portal venous inflow to the liver, wider indications (including for some Child-Pugh class C patients), low mortality and complication rates, improved postoperative quality of life, and applicability in primary care hospitals. These factors make devascularization the most widely used surgical option (85% of cases).
Disadvantages are persistent portal hypertension and a higher rate of rebleeding compared to shunt surgeries.
Common methods include paraesophagogastric devascularization, gastric devascularization, distal esophageal transection, gastric fundus transection, and esophagogastric resection. Among these, splenectomy with paraesophagogastric devascularization is the most commonly performed. This procedure thoroughly interrupts abnormal collateral circulation, including the coronary vein (with gastric, esophageal, and ectopic branches), short gastric veins, posterior gastric veins, and left inferior phrenic veins. It also involves ligation and division of accompanying arteries, preserving hepatic portal venous inflow.
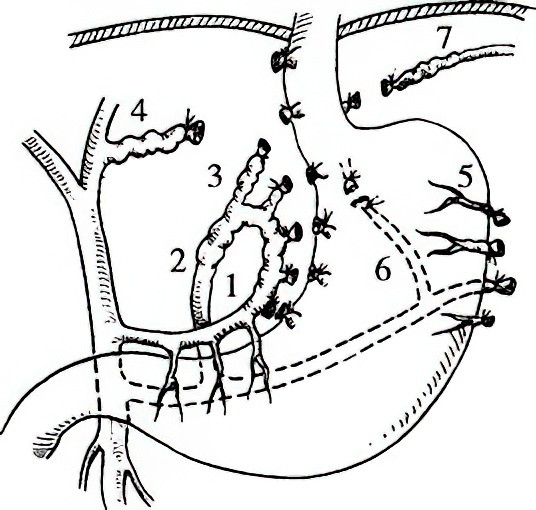
Figure 5 Schematic diagram of paraesophagogastric devascularization
1, Gastric branch
2, Esophageal branch
3, High esophageal branch
4, Ectopic high esophageal branch
5, Short gastric vein
6, Posterior gastric vein
7, Left inferior phrenic vein
This procedure is suitable for patients requiring elective surgery due to a history of variceal bleeding, those needing emergency surgery for active variceal rupture, patients with failed shunt surgery or non-surgical approaches who are not candidates for further shunting, and in cases requiring prophylactic surgery. Laparoscopic devascularization has been widely adopted in clinical practice for its reduced bleeding and trauma compared to conventional open surgery.
Combination Surgeries
Combination procedures integrate the benefits of selective shunting and devascularization by reducing high portal blood flow while preserving partial hepatic portal venous inflow. These procedures aim to balance the strengths of both methods for improved outcomes. However, they are more technically demanding, carry higher risks of complications, and are only appropriate for patients with relatively preserved liver function.
Splenomegaly and Hypersplenism
In portal hypertension, abnormal immune function of the spleen contributes to the progression of liver disease. Splenectomy is the most effective treatment for hypersplenism, as it lowers portal pressure and slows the progression of liver disease. Most devascularization procedures and some shunt surgeries include splenectomy as part of the treatment. Other treatments, such as splenic radiofrequency ablation and splenic artery embolization, have uncertain effectiveness for hypersplenism and are associated with higher complication rates. These methods are mostly used for patients unwilling or unable to undergo surgery.
Refractory Ascites
Refractory ascites refers to large and persistent amounts of ascitic fluid that do not respond to standard treatments, such as diuretics or albumin supplementation. Treatment options for refractory ascites include terlipressin therapy, paracentesis with external drainage, TIPS (transjugular intrahepatic portosystemic shunt), peritoneovenous shunt, or subcutaneous ascitic shunt placement. In cases where spontaneous bacterial peritonitis exists, adding antibiotics can improve the treatment outcome.
Underlying Liver Disease
The majority of portal hypertension cases are caused by viral hepatitis-associated cirrhosis, with significant liver function impairment. Antiviral therapy and liver protection strategies are essential throughout the treatment process. In cases of severe cirrhosis with poor liver function that does not improve with medical therapy, liver transplantation is recommended. Liver transplantation addresses both the liver pathology and the hemodynamic abnormality in the portal venous system, making it the most definitive treatment available. However, the scarcity of donor livers, lifelong immunosuppressive therapy, and high costs remain significant drawbacks.
From the above, it can be seen that the etiology and pathological changes of portal hypertension are diverse and complex. The variety of treatment options each has its own advantages and disadvantages. Achieving improved clinical outcomes and better patient prognoses requires selecting a scientifically sound and individualized treatment plan based on specific circumstances.